Nutricia has advised that they are continuing to experience supply issues with Allerpro Syneo Stages 1 and 2, Pepti Junior and Neocate Junior Unflavoured (see table below). Nutricia has also advised that there is good supply for Neocate Gold and Neocate Junior Vanilla.
If you are affected by formula supply issues, alternative formula and milk products are listed on the ASCIA website: www.allergy.org.au/hp/papers/guide-for-milk-substitutes-cows-milk-allergy
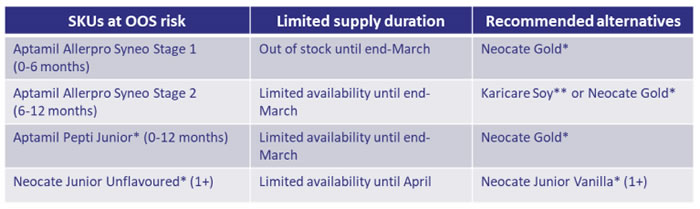
*Please note, these products need authority from the PBS or can be prescribed in consultation with a specialist i.e., paediatric allergist / immunologist or gastroenterologist. This consultation can take the form of a phone call or email between the GP / paediatrician and the specialist and, the patient must also have an appointment to see the specialist. They have also made allowances for waiting lists to see specialists.
**Not suitable for infants with suspected or confirmed soy allergy.
For supply of Karicare Soy, please note itis available in Woolworths, Coles and Chemist Warehouse stores nationwide and in www.nutriciastore.com.au
If pharmacies are having difficulty ordering Neocate Gold or Neocate Junior Vanilla from their usual wholesalers, the following options are available:
- Local pharmacy to order directly from Nutricia: Patients can ask their local pharmacy to contact Nutricia directly on 1800 889 480 or This email address is being protected from spambots. You need JavaScript enabled to view it. to place an order using a credit card.
- Order online - Nutricia have partnered with some pharmacies that offer e-script fulfilment and interstate delivery. Patients just need to send their scripts via email to the following pharmacies:
• Better Value Springvale, VIC - This email address is being protected from spambots. You need JavaScript enabled to view it.
• Better Value Box Hill, VIC - This email address is being protected from spambots. You need JavaScript enabled to view it.
• UFS Dispensaries, VIC - This email address is being protected from spambots. You need JavaScript enabled to view it.
Where CMPA is no longer suspected or has resolved, you may also consider the following products for the management of other feeding issues:
• Aptamil Gold+ Reflux – thickened formula with prebiotics for the dietary management of reflux or regurgitation
• Aptamil Gold+ Colic & Constipation –partially hydrolysed formula, low in lactose with prebiotics for the dietary management of colic and/or constipation
• Aptamil Prosyneo Sensitive – partially hydrolysed formula with prebiotics and probiotics
For supply of these products, please note that they are available in Woolworths, Coles, and Chemist Warehouse nationwide and in www.nutriciastore.com.au
For any additional concerns or urgent queries, GPs, Allergy and Paediatric Specialists can contact their Nutricia Representative or the Nutricia Careline team on any of the following:
Phone: 1800 438 500
Email: This email address is being protected from spambots. You need JavaScript enabled to view it.
Live chat: www.aptanutrition.com.au
Danone Nutricia has confirmed that there is good supply for Neocate Gold and Neocate Junior Vanilla. All Neocate formulas are specialised amino acid-based medical nutrition for the dietary management of infants and children with severe/complex cow's milk allergy, multiple food protein allergy and other indications where an amino acid diet is recommended by a healthcare professional.
Options to order Neocate products are as follows:
- For pharmacies and hospitals:
• Order directly from Nutricia: Patients can ask their local pharmacy to contact Nutricia directly on 1800 889 480 or This email address is being protected from spambots. You need JavaScript enabled to view it. to place an order using a credit card.
• Order via their usual wholesaler
- For patients:
• Purchase via their usual pharmacy
• Order online - Nutricia have partnered with some pharmacies that offer e-script fulfilment and interstate delivery. Patients just need to send their scripts via email to the following pharmacies:
o Better Value Springvale, VIC - This email address is being protected from spambots. You need JavaScript enabled to view it.
o Better Value Box Hill, VIC - This email address is being protected from spambots. You need JavaScript enabled to view it.
o UFS Dispensaries, VIC - This email address is being protected from spambots. You need JavaScript enabled to view it.
Continue reading
![]() Allergen Immunotherapy 17 March 2022 Invitation162.73 KB
Allergen Immunotherapy 17 March 2022 Invitation162.73 KB
