Position Statement - Immunoglobulin Replacement Therapy (IRT) for Primary Immunodeficiency (PID)
The aim of this document is to improve health outcomes for people with inborn errors of immunity (IEI), including primary immunodeficiencies (PID), by:
- Assisting GPs, paediatricians and other medical specialists to recognise the early signs of PID and refer patients to a clinical immunologist to confirm diagnosis and initiate treatment, including immunoglobulin replacement therapy (IRT) if required.
- Increasing awareness of IRT options for patients with PID, which is given as intravenous immunoglobulin (IVIg) or subcutaneous immunoglobulin (SCIg), and the pros and cons of IVIg versus SCIg.
![]() ASCIA HP Position Statement IRT for PID 2025347.36 KB
ASCIA HP Position Statement IRT for PID 2025347.36 KB
Contents
- Overview of PID
- Early recognition and referral of patients with PID
- Warning signs of PID
- IRT for PID
- IVIg or SCIg
- Resources
This document is based on expert opinion, consensus and publications, and reviewed by the ASCIA IRT/PID Working Party. This document is a source for ASCIA IRT e-training for health professionals which is available at https://traininghp.ascia.org.au.
Key points
- Inborn errors of immunity (IEI), including PID are a diverse group of more than 550 potentially serious, chronic illnesses due to inherited absence or dysregulation of parts of the immune system.
- Due to their rarity, delay in the diagnosis of PID is common, increasing the risk of further complications and reduced survival rates. As well as the recognition of warning signs, improved access to specialist clinical and diagnostic laboratory services is required to improve the early diagnosis and treatment of PID.
- Early and correct diagnosis of PID leads to appropriate treatment, including IRT, which improves quality and length of life. The aim of this treatment is to replace immunoglobulin to maintain normal Immunoglobulin G (IgG) levels, with the dose used individualised for each patient.
- IRT is the standard of care for patients with antibody deficiency due to a PID disease. It should be readily available to these patients while under the active care of a clinical immunology/allergy specialist with support from specialist medical, nursing and allied health professionals.
- IRT can be given as IVIg or SCIg and pharmacokinetics differ according to administration route. The choice of route (IVIg or SCIg) is dependent on multiple factors, including patient preference, medical conditions and lifestyle. The preferred route may vary at different times during a patient’s life.
1. Overview of PID
Inborn errors of immunity (IEI), including primary immunodeficiencies (PID), are a diverse group of more than 550 potentially serious, chronic illnesses due to inherited absence or dysregulation of parts of the immune system. Symptoms often appear in childhood, but many can first occur in adult life.
PID can lead to reduced quality of life and life expectancy due to recurrent, chronic or severe infections, swellings, autoimmune and inflammatory problems, and are a significant health burden.
PID are different from acquired immunodeficiencies (also known as secondary immunodeficiencies), which may be due to malignancy, cancer treatments, immunosuppressive medications, autoimmune diseases, or infections such as the human immunodeficiency virus (HIV), which causes acquired immunodeficiency syndrome (AIDS).
There are six main types of primary immunodeficiencies that affect the immune system in different ways:
|
Note: A published classification of IEI has been developed by the International Union of Immunological Societies (IUIS), which is regularly modified.
Research and advances in therapies have resulted in improved health and a longer life for people with PID. There are currently six main types of treatment options depending on the type of PID:
|
The focus of this document is IRT (SCIg or IVIg) for patients with PID.
For further information about the types of PID and treatments refer to Appendices A and B in the ASCIA Immunodeficiency Strategy for Australia and New Zealand
2. Early recognition and referral of patients with PID
Due to their rarity, delay in diagnosis of PID is common, increasing the risk of further complications and reduced survival rates:
- For infants and very young children with severe PID, diagnostic delay leads to severe complications due to infections and early death.
- Early diagnosis is vital for severe PID, to allow curative treatment such as urgent HSCT, also known as bone marrow transplant.
- For older children and adults with PID where curative treatment is not currently possible, delay in diagnosis can be associated with:
- Infections, resulting in possible organ damage.
- Increased morbidity.
- Reduced life expectancy.
Early and correct diagnosis will lead to appropriate treatment, including IRT, which improves quality and length of life. This requires support from expert multi-disciplinary teams comprising of specialist medical, nursing and allied health professionals.
As well as the recognition of warning signs listed in this document, access to specialist clinical and diagnostic laboratory services is required to improve early diagnosis and treatment.
With targeted resources, patients with PID can be spared unnecessary interventions, and instead utilise available medical treatments to maximise their opportunities to lead productive and healthy lives.
The role of GPs, paediatricians and physicians in identifiying and managing patients with PID
GPs, paediatricians and adult medicine physicians, particularly respiratory medicine physicians and gastroenterologists, have an important role in identifying and managing patients with PID including:
- Recognition of early symptoms and signs of PID.
- Appropriate investigation and interpretation of test results.
- Appropriate and timely referral to a clinical immunologist.
- Appropriate follow up care in conjunction with a clinical immunologist.
- Management of general health issues, particularly assessment of growth and development in children in patients with PID.
When should patients be referred to a clinical immunologist?
Patients with suspected PID should be referred to a clinical immunologist when:
- They have early warning signs of PID.
- Results of initial testing suggests PID.
- Results of initial testing are confusing and diagnosis is unclear.
- Results do not confirm PID but there remains a high clinical suspicion for PID.
3. Warning signs of PID
Early diagnosis of other PID is important, since delayed treatment results in complications that can be chronic or life threatening.
Warning signs of PID are listed below. However, there is a broader range of symptoms and signs as some PID patients may not present with recurrent and severe infection but develop other features such as autoimmunity, autoinflammation or neoplasiaIf clinical concern exists patients should be referred to a clinical immunologist for further assessment.
|
CHILDREN |
ADULTS |
|
Four or more ear infections within one year |
Two or more ear infections (otitis media) within one year |
|
Two or more serious sinus infections within one year |
Two or more sinus infections in one year in the absence of allergies |
|
Two or more pneumonias within one year |
Recurrent pneumonia |
|
Recurrent, deep skin or organ abscesses |
Recurrent, deep skin or organ abscesses |
|
Two or more deep seated infections such as sepsis, meningitis or cellulitis |
Infection with normally harmless tuberculosis-like bacteria |
|
Persistent thrush in the mouth, skin or elsewhere after age one |
Persistent thrush or fungal infection on skin or elsewhere |
|
Two or more months on antibiotics with little effect |
Persistent or recurrent viral infections (warts, herpes, EBV) |
|
Need for intravenous antibiotics to clear infections |
Need for intravenous antibiotics to clear infections |
|
Failure to gain weight, grow at a normal rate, or chronic diarrhea |
|
|
Family history of PID |
Family history of PID |
This table is adapted from the ten warning signs developed by the Jeffrey Modell Foundation www.info4pi.org
4. IRT for PID
In people who have primary antibody (immunoglobin) deficiencies, immunoglobulin replacement therapy (IRT):
- Is the standard of care.
- Is used to maintain Immunoglobulin G (IgG) levels.
- Greatly improves health and quality of life.
- Is often required lifelong, to prevent organ damage due to recurrent infections, which can be life saving.
IRT should be given under the supervision of a medical specialist trained in the care of patients with primary antibody deficiencies.
Access to IRT is guided by clear prescribing criteria to ensure clinically appropriate and economical use of immunoglobulin products.
All immunoglobulin products approved for use in Australia and New Zealand:
- Are made from pooled plasma from many healthy human donors, which is screened for hepatitis B, hepatitis C and HIV.
- Are treated with additional viral inactivation steps such as heat treatment, enzyme treatment, detergent treatment and nanofiltration.
- Contain 97-98% IgG specific antibodies against a broad spectrum of bacterial and viral pathogens, with traces of IgM and IgA.
- Are plasma derived products and therefore a limited resource. Prescribing a dose that uses a partial vial results in unnecessary wastage so prescribers must ensure that doses are rounded to the full vial size. Vial sizes vary between products and this must be taken into account.
- Are available to patients meeting the prescribing criteria under the active care and follow up of a clinical immunology/allergy specialist.
Immunoglobulin replacement therapy (IRT) can be given as IVIg or SCIg and pharmacokinetics differ according to administration route.
Both IVIg and SCIg:
- Are effective at reducing infections and hospitalisations.
- Preserve organ function and reduce long term damage from recurrent infections.
- Are associated with significant benefits to patient quality of life.
- Improve the lifespan of patients with PID.
- Have advantages and disadvantages, and the preferred route may vary during a patient’s life.
Multiple brands of IRT products are available through the National Blood Authority (NBA) and the New Zealand Blood Service (NZBS). Rates of administration may vary for different products.
5. IVIg or SCIg
Intravenous immunoglobulin (IVIg) replacement therapy:
- Is usually administered approximately monthly (three to four weekly) in hospital.
- Leads to a high peak of IgG after infusion.
- Levels decrease rapidly over a few days then slowly decrease over the next few weeks.
- Does not require patient training as it is usually hospital based.
Most side effects are mild and self-limiting and include headache, fever, chills, nausea, and fatigue. The frequency of side effects may be linked to the rate of infusion as more side effects are seen with faster infusion rates.
Serious adverse events are rare but include anaphylaxis, aseptic meningitis, renal impairment and thrombosis.
Advantages and disadvantages of IVIg therapy
|
|
Advantages |
Disadvantages |
|
IVIg |
|
|
This table is an updated version of the original table that was adapted from APIIEG.
Subcutaneous immunoglobulin (SCIg) replacement therapy:
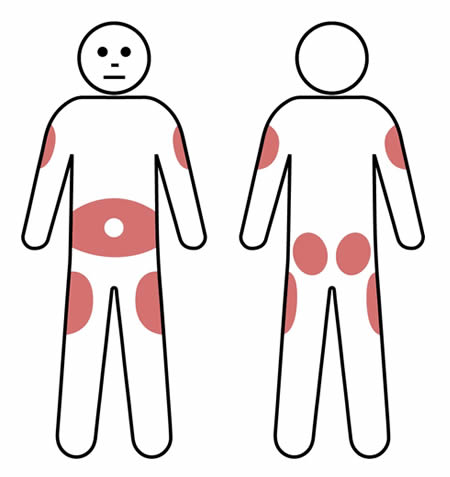 Requires frequent administration (ranging from 1-3 times per week to once a fortnight) by patients or carers at home.
Requires frequent administration (ranging from 1-3 times per week to once a fortnight) by patients or carers at home.- Involves slow diffusion of IgG from subcutaneous tissue.
- Is associated with more consistent IgG levels due to frequent administration. Is administered at multiple injection sites according to personal preference, usually in the lower abdomen. However, the outer edge of the thigh or back of the upper arm can also be used, as shown in this image.
Systemic reactions to SCIg are much less common compared with IVIg.
Local injection site reactions such as redness, itching, swelling, or discomfort are common, but improve with time.
Advantages and disadvantages of SCIg therapy
|
|
Advantages |
Disadvantages |
|
SCIg |
|
|
This table is an updated version of the original table that was adapted from APIIEG
Several factors affect the choice of IVIg or SCIg therapy
The decision to have immunoglobulin replacement therapy (IRT) administered as intravenous immunoglobulin (IVIg) or subcutaneous immunoglobulin (SCIg) depends on each patient’s situation including medical history, response to treatment, compliance with therapy and lifestyle.
Factors that may affect the choice of route for IRT (IVIg or SCIg) include:
- Patient preference and satisfaction - This plays an important role in treatment decisions, particularly as some peopple with antibody defiencies require lifelong IRT.
- Availability - There is a small number of conditions where SCIg has been approved for use under the national blood supply arrangements in Australia. Eligibility is dependant on the patient’s specific condition.
- Resourcing of SCIg products and consumables - All consumables are provided at no cost to patients who are eligible for SCIg under the national blood arrangements. Some patients may prefer to use a pump. The arrangements for provision of pumps is different in each state and territory and there may be a cost involved. Health professionals should familiarise themselves with local policies and procedures to be able to correctly inform their patients.
- Other medical conditions - SCIg therapy may be contraindicated in some patients with severe thrombocytopenia, bleeding disorders or for patients on anticoagulation therapy and may also be problematic for patients with widespread eczema.
- Less frequent infusion procedures may be preferred for some young patients.
The role of nurses - educating and supporting people on immunoglobulin replacement therapy (IRT)
Nurses play a vital role in educating and supporting people who are being treated with immunoglobulin products. To assist with this process, ASCIA SCIg Nurse Competency and Patient Training Checklists are available at www.allergy.org.au/hp/papers/ascia-scig-competency-training-checklists
It is important that nurses have evidence-based information on:
- Access to and supply of immunoglobulin products.
- Administration of these products including storage and cold chain management.
- The role of immunoglobulins in treating a range of immunology, haematology and neurology conditions.
- Recognising and managing side effects or adverse reactions.
The role of medical specialists - prescribing immunoglobulin replacement therapy (IRT)
In addition to complying with approved uses of IRT, medical specialists assess:
- Which patients are eligible for IRT and are likely to benefit.
- Response to therapy.
- When to consider trial off therapy, if appropriate.
Prescribing immunoglobulin replacement therapy (IRT) by medical specialists in Australia
The approved uses for immunoglobulin products are set out in the Criteria for the Clinical Use of Immunoglobulin in Australia https://www.criteria.blood.gov.au/CheckEligibility which are funded through the National Blood Agreement.
To access immunoglobulin under the Agreement, a medical officer is required to submit an application through the National Blood Authority (NBA) national online system BloodSTAR (Blood System for Tracking Authorisations and Reviews).
LifeBlood, via BloodSTAR, manages the authorisation request and review process and ensures that access to Ig products is consistent with the Criteria. Immunoglobulin products are funded by the government and provided to eligible patients at no direct cost.
Some patients who are ineligible to access immunoglobulin products may be able to access them through a Jurisdictional Direct Order, at a cost to the approved health provider, or directly from suppliers, at a personal cost.
Prescribing immunoglobulin replacement therapy (IRT) by medical specialists in New Zealand
In New Zealand individual clinicians are required to seek authorisation from New Zealand Blood Service (NZBS) for the administration of immunoglobulin to individual patients.
Where treatment is managed under the care of a District Health Board (DHB) approval must also be gained from that DHB’s local immunoglobulin authority (e.g. immunoglobulin committee).
For further information go to www.nzblood.co.nz/Clinical-information/Transfusion-medicine/Information-for-Health-Professionals/Request-forms
The role of pharmacists or blood bank – dispensing immunoglobulin replacement therapy (IRT)
Dispensers (blood banks or pharmacists) of immunoglobulin products support the safe use and quality management of these products in health services, by ensuring that the right immunoglobulin products are ordered and dispensed at the right time.
Treatment Summary for Patients on Immunoglobulin Therapy
 The ASCIA Treatment Summary for patients is available at www.allergy.org.au/hp/papers/ascia-transfer-care-plan-irt
The ASCIA Treatment Summary for patients is available at www.allergy.org.au/hp/papers/ascia-transfer-care-plan-irt
This plan has been developed as a medical document to be completed by an immunology or nurse specialist, when a patient is transitioning from:
- Paediatric to adult medical care
- One region to another
- IVIg to SCIg
- SCIg to IVIg
6. Resources
Further information is available from:
BloodSafe eLearning https://learn.bloodsafelearning.org.au/categories#immunoglobulin-courses
National Blood Authority www.blood.gov.au/
ASCIA www.allergy.org.au/immunodeficiency
ASCIA - Patients and carers: www.allergy.org.au/patients/immunodeficiencies
ASCIA - Health professionals: www.allergy.org.au/hp/papers/immunodeficiency
ASCIA – Online training: https://traininghp.ascia.org.au/
Patient support
Australian Primary Immunodeficiency Patient Support (AusPIPS Inc) www.auspips.org.au
Immune Deficiencies Foundation Australia (IDFA) www.idfa.org.au
Immune Deficiencies Foundation New Zealand (IDFNZ)www.idfnz.org.nz
© ASCIA 2025
Content updated May 2025
ASCIA is the peak professional body of clinical immunology/allergy specialists in Australia and New Zealand.
ASCIA resources are based on published literature and expert review, however, they are not intended to replace medical advice. The content of ASCIA resources is not influenced by any commercial organisations.
For more information go to www.allergy.org.au
To donate to immunology/allergy research go to www.allergyimmunology.org.au
