ASCIA HP Checklist Anaphylaxis Pharmacists 2024120.74 KB
ASCIA HP Checklist Anaphylaxis Pharmacists 2024120.74 KB
This document has been developed by ASCIA, the peak professional body of clinical immunology/allergy specialists in Australia and New Zealand. ASCIA information is based on published literature and expert review, is not influenced by commercial organisations and is not intended to replace medical advice. For patient or carer support contact Allergy & Anaphylaxis Australia or Allergy New Zealand.
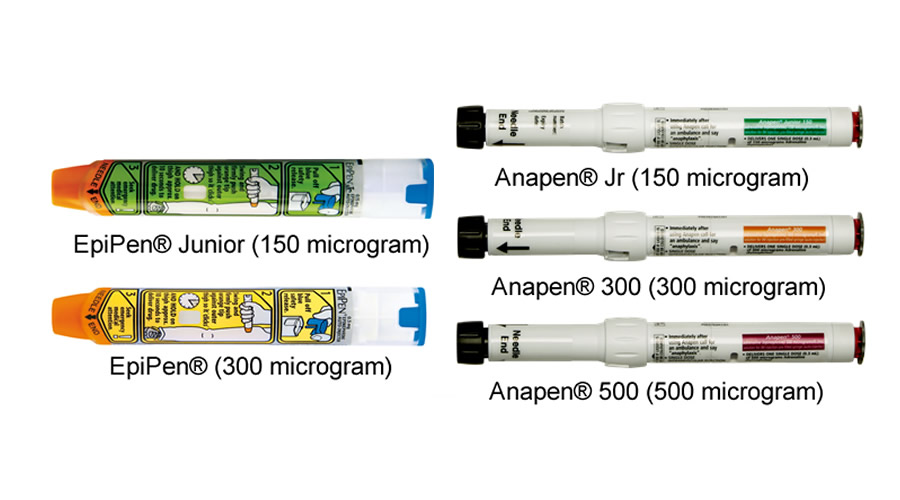
 There are two brands of adrenaline injectors, EpiPen® and Anapen®:
There are two brands of adrenaline injectors, EpiPen® and Anapen®:
- EpiPen® (300 mcg) and EpiPen® Jr (150 mcg) are available in Australia on the PBS, and New Zealand (Pharmac listed since 1 February 2023).
- Anapen® 500, Anapen® 300 and Anapen® 150 are available in Australia on the PBS.
Adrenaline injectors are prescribed as follows:
- 150 mcg devices for children weighing 7.5 to 20kg.
- 300 mcg devices for children weighing over 20kg and adults, including pregnant women.
- 500 mcg or 300 mcg devices for children weighing over 50kg and adults, including pregnant women.
- Dispense prescribed adrenaline (epinephrine) injectors
- Dispense prescribed injectors and check that the dose is appropriate (see above).
- Check that the patient has been prescirbed two devices, which are rebated by the PBS in Australia and New Zealand.
- Remind the customer (patient and/or carer) to check and note the expiry date of their injector/s.
- Check that the patient has an ASCIA Action Plan for Anaphylaxis (RED) that should have been completed by their doctor or nurse practitioner.
- Reinforce the need for the patient to always carry their adrenaline injector and ASCIA Action Plan.
- Check that the patient’s asthma is well managed and refer them back to their doctor or nurse practitioner if further action is required. Patients at risk of severe allergic reactions (anaphylaxis) who have asthma that is not well controlled are at increased risk of fatal anaphylaxis.
- Dispense non-prescribed adrenaline injectors
- Dispense adrenaline injector/s to customers (patients/carers or organisations) for use in addition to prescribed devices or in first aid kits. Non-prescribed devices are not rebated by PBS or Pharmac.
- Remind the customer to check and note the expiry date of their adrenaline injector/s.
- Check that the customer has an ASCIA First Aid Plan for Anaphylaxis (ORANGE) that should be stored with the non-prescribed device/s. These plans are available at www.allergy.org.au/anaphylaxis
- Provide adrenaline injector advice, education and training
- Teach the customer how to use their prescribed or non-prescribed adrenaline injector/s using a trainer device. Trainer devices are available from the supplier or patient organisations (Allergy & Anaphylaxis Australia or Allergy New Zealand).
- Educate the customer about correct storage of adrenaline injectors, away from excessive heat or cooling.
- Ensure the customer understands that adrenaline is the first line treatment for anaphylaxis and that antihistamines should not be used for the treatment for anaphylaxis. If antihistamines are used to treat mild to moderate allergic reactions, only non-sedating antihistamines should be used.
- Be prepared for an anaphylaxis emergency
- Train staff to recognise the signs and symptoms of anaphylaxis, how to position the patient, how to administer adrenaline injectors and to phone an ambulance immediately afterwards.
- Display ASCIA Action First Aid Plans for Anaphylaxis to assist staff in recognising and responding to an anaphylaxis emergency.
For adrenaline injector supply updates check www.allergy.org.au/members/adrenaline-autoinjector-availability
© ASCIA 2024
Content updated March 2024
For more information go to www.allergy.org.au/hp/anaphylaxis
To support allergy and immunology research go to www.allergyimmunology.org.au/donate
![]() ASCIA PBS adrenaline device authority112.5 KB
ASCIA PBS adrenaline device authority112.5 KB![]() ASCIA PBS adrenaline device authority26.32 KB
ASCIA PBS adrenaline device authority26.32 KB

 This document has been developed by
This document has been developed by