Allergic Rhinitis Clinical Update
This document has been developed by ASCIA, the peak professional body of clinical immunology/allergy specialists in Australia and New Zealand. ASCIA information is based on published literature and expert review, is not influenced by commercial organisations and is not intended to replace medical advice.
For patient or carer support contact Allergy & Anaphylaxis Australia or Allergy New Zealand.
![]() ASCIA HP Clinical Update Allergic Rhinitis 2024649.94 KB
ASCIA HP Clinical Update Allergic Rhinitis 2024649.94 KB
Contents
- Key Points
- Overview
- Clinical Assessment
- Aeroallergen Minimisation
- Medications for Allergic Rhinitis
- Allergen Immunotherapy
References are listed on the ASCIA website www.allergy.org.au/hp/papers/references-allergic-rhinitis
Key Points
 Minimising exposure to confirmed allergens may assist in reducing symptoms of allergic rhinitis.
Minimising exposure to confirmed allergens may assist in reducing symptoms of allergic rhinitis.- Results of allergy tests should always be considered with a patient’s clinical history. Positive tests do not automatically prove the allergen is causing the symptoms.
- Intranasal corticosteroids sprays or combined intranasal/antihistamine sprays are recommended first line treatments.
- Patients should be instructed on the correct use of treatments by providing an ASCIA Treatment Plan for Allergic Rhinitis
- The management of asthma is important in the effective treatment of allergic rhinitis.
- If patients are allergic to pollen, recommend that they stay indoors during, before and after thunderstorms in pollen seasons, or on windy days, and use preventer treatments.
- Referral to a clinical immunology/allergy specialist to initiate allergen immunotherapy should be considered when severe or inadequately controlled allergic rhinitis persists.
- Allergen immunotherapy is effective in reducing the frequency and severity of symptoms of allergic rhinitis.
Overview
Allergic rhinitis is a local IgE mediated allergic condition, a response of the nasal airways to inhaled allergens.
Common aeroallergen triggers of allergic rhinitis are house dust mites, grass, tree or weed pollen, animal dander, and mould spores.
Allergic rhinitis, commonly referred to as hay fever:
- Is the most common allergic disorder in Australia and New Zealand.
- Is often underdiagnosed, undertreated, and sub-optimally self-treated.
- Can have a significant impact on sleep, concentration, learning and daily function, and affect childhood behaviour and development.
- Can be effectively managed and treatment is important for management of asthma.
Brief history
The term “hay fever” was used to describe seasonal allergic rhinitis from the late 18th century, when the belief was that the effluvium from new hay was the main cause of symptoms. In the late 19th century, Dr Charles Blackley discovered that pollen from wind pollinated trees, grasses and weeds was the major cause of seasonal allergic rhinitis. In 1906 the term “allergy” was first used, derived from the “allos” meaning “other” or a deviation from the original state. This was combined with “rhinitis” meaning “inflammation of the nose”.
Allergic rhinitis is common in Australia and New Zealand
Based on self-reports, almost one in four Australians (23.9% of the population) had allergic rhinitis as stated in the Australian Bureau of Statistics (ABS) National Health Survey 2023.
In the 2018 survey it was found to be most common between 15-54 years of age (peak between 35-44 years of age). Children were less likely to have allergic rhinitis (10%).
Allergic rhinitis is a local IgE mediated allergic condition
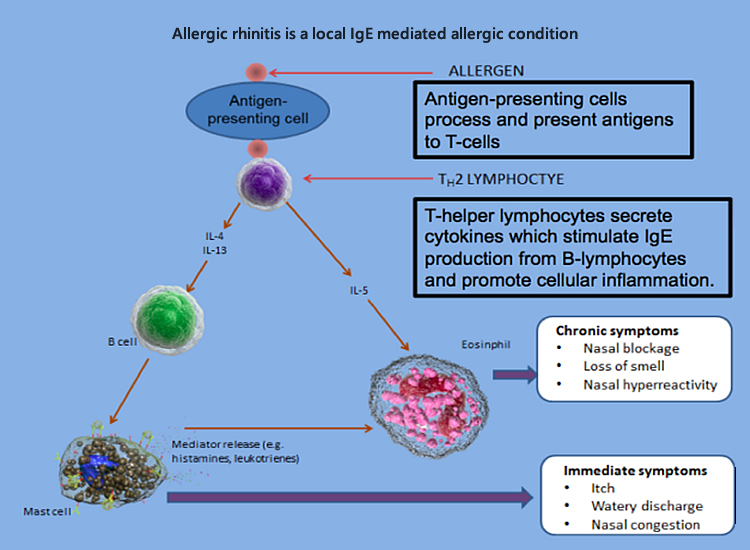
Symptoms of allergic rhinitis include:
- Sneezing, itchy nose, sniffing, upward rubbing of the nose.
- Clear rhinorrhea.
- Nasal obstruction/congestion such as nasal speech, mouth breathing, snoring.
- Itchy throat, a frequent need to clear the throat.
Symptoms may be confused with recurrent upper respiratory tract infections.
Allergic conjunctivitis presents with watery, itchy eyes and may occur in conjunction with allergic rhinitis or in isolation.
Classification
Clinical presentation of allergic rhinitis can be classified by timing of allergen exposure, duration and severity of symptoms.
Timing of allergen exposure is defined as:
- Perennial - year-round symptoms triggered by indoor allergens such as dust mite, animal dander, and/or moulds.
- Seasonal - symptoms worsen during spring or summer and are often triggered by the pollens of grasses, weeds, or trees as well as moulds.
- Perennial with seasonal exacerbations - Some patients may also be sensitised to many different ‘seasonal’ allergens and present with perennial symptoms, with seasonal exacerbations. Seasonal allergens present year-round in certain regions.
- Occupational - triggered by chemicals, irritants, or allergens in the workplace. Symptoms improve when away from the workplace.
Duration and severity of symptoms is defined as:
- Intermittent: <4 days/week or <4 weeks.
- Persistent: >4 days/week or >4 weeks.
- Mild: Normal sleep, no impairment of daily activities, normal work, or school performance.
- Moderate-severe: One or more of the following: abnormal sleep, impairment of activities, abnormal work or school performance, troublesome symptoms.
Allergic rhinitis can coexist with other conditions
Allergic rhinitis can coexist with a range of other conditions including:
- Asthma
- Nasal polyps
- Eustachian tube dysfunction
- Oral allergy syndrome
- Conjunctivitis
- Non-allergic rhinitis
Asthma and allergic rhinitis
United airway disease is the concept that allergic rhinitis and asthma are upper and lower respiratory tract manifestations of the same inflammatory process. Inhalation of aeroallergen via the nose may contribute to inflammation in the lungs. Allergic rhinitis is a risk factor for subsequent asthma development.
Patients with either asthma or allergic rhinitis should be assessed for coexistent disease, because:
- 50-80% of patients with asthma have allergic rhinitis.
- 20-30% of patients with allergic rhinitis have asthma.
Effective treatment of allergic rhinitis may improve asthma severity/control.
Thunderstorm asthma
Thunderstorm asthma is usually due to thunderstorms with rapid changes in wind, temperature and humidity. This causes pollen grains to absorb moisture, burst open and release large amounts of small pollen allergen particles. These particles penetrate the small airways of the lung, which can be fatal if medical treatment is delayed.
Even on days with high pollen counts, not all thunderstorms trigger thunderstorm asthma.
Not everyone affected by Australian thunderstorm asthma has previously experienced this condition. However, they have usually had severe allergic rhinitis and are allergic to ryegrass pollen. Other allergens such as fungal spores can also affect some people during a thunderstorm.
ASCIA Treatment Plan for Allergic Rhinitis includes information on thunderstorm asthma.
AusPollen Apps are available at www.pollenforecast.com.au and these aim to provide accurate and easily accessible information on local pollen counts.
Chronic rhinosinusitis with nasal polyps
Chronic rhinosinusitis with nasal polyps (CRSwNP) may coexist with allergic rhinitis. It is defined as inflammation of the paranasal sinuses for more than 12 weeks.
CRSwNP is present in 2-4% of the adult population.
- Aspirin hypersensitivity is common in people with polyposis and asthma (Samter’s triad).
- Consider polyps if a patient presents with persistent nasal obstruction and/or anosmia.
- Large polyps may be seen on anterior rhinoscopy.
- Consider referral to clinical immunology/allergy specialist or ear, nose and throat (ENT) surgeon for treatment with biologic medications or surgery.
For more information on chronic rhinosinusitis with nasal polyps diagnosis, treatment and management, refer to the ASCIA CRSwNP Position Paper
Normal nose, inferior turbinate hypertrophy and polyp as examined on anterior rhinoscopy

Ears and allergic rhinitis
Allergic rhinitis may contribute to ear symptoms such as fullness, blockage, and/or hearing loss due to mucous and oedema in the Eustachian tube. Blockage of the Eustachian tube results in negative middle ear pressure and middle ear effusion (glue ear).
Young children are more prone as they have Eustachian tubes with a smaller diameter, and an increased predisposition to recurrent upper respiratory infections.
Oral allergy syndrome and allergic rhinitis
Certain fresh vegetables and fruits cause oral symptoms of itch and swelling in some patients, known as oral allergy syndrome (OAS), also known as pollen food syndrome. Serious OAS reactions are rare. OAS most commonly occurs in people with asthma or allergic rhinitis who are sensitised to inhaled tree, grass or weed pollens, which contain proteins that are similar to proteins found in foods.
Some pollen and food allergens share common allergenic proteins, which are known as cross reactive proteins. This means that in some people with pollen allergy, their immune system confuses a food protein with a pollen protein, resulting in OAS.
Diagnosis of OAS may be confirmed by a clinical immunology/allergy specialist using fresh food in a skin prick test.
Dietary restrictions are not recommended for allergic rhinitis
- There is no evidence that allergic rhinitis is due to food allergies, although conditions may coexist.
- Food elimination is not recommended unless there is a confirmed allergy, and has potential for serious nutritional consequences, especially in young children.
- Restricting cow’s milk (dairy) products is often popular, even if there is no confirmed food allergy, but studies do not show any change in mucus production following dietary modification.
Allergic rhinitis management
The role of the medical practitioner or other health professional is to:
- Provide diagnosis.
- Diagnose and manage comorbid conditions such as asthma and allergic conjunctivitis.
- Initiate treatment if required.
- Educate the patient on:
- Strategies to minimise aeroallergen exposure.
- How to use intranasal sprays correctly.
- Potential side effects of medications.
- Refer to a clinical immunology/allergy specialist when indicated, for example to initiate allergen immunotherapy. Information on how to Locate a Specialist is on the ASCIA website.
Allergic rhinitis management during pregnancy
- Up to 20% of pregnant women develop symptoms of rhinitis, typically in second trimester, improving two weeks after delivery.
- Medications for allergic rhinitis should only be used during pregnancy if the benefit to the mother justifies the potential risk to the foetus.
- There are few well-controlled clinical studies in pregnant women examining the safety of many of the medications used in allergic rhinitis.
- Ideally pharmacotherapy should be avoided in the first trimester of pregnancy. However, there are some oral antihistamines and intranasal corticosteroid sprays with an “A” category used many pregnant women without any proven increase in harmful effects on foetus.
- Saline nasal irrigation is safe in pregnancy.
- Refer to MIMS (Australia or New Zealand) or go to the Prescribing medicines in pregnancy database before prescribing any medication in pregnancy.
Allergic rhinitis management during lactation
Recommend taking medication after feeding the infant to minimise any potential infant exposure.
|
Safety Consideration
|
Medication
|
|
Considered safe
|
Saline nasal treatments
Intranasal ipatropium (anti-cholinergic)
Non-sedating oral antihistamines
Intranasal corticosteroids
Intranasal decongestants
Intranasal antihistamines
|
|
Crosses into breast milk (recommend not to use)
|
Oral decongestants
|
Clinical Assessment
Patient's Clinical History
|
Important points to consider
|
|
|
Timing of symptoms
|
Perennial (year-round) and/or seasonal
|
|
Impact of symptoms
|
Mild (no effect on day-day function) or moderate-severe (impaired day-day function)
|
|
Frequency of symptoms
|
Intermittent (< 4 days/week or < 4 weeks) or persistent (≥ 4 days/week and for ≥ 4 weeks)
|
|
Triggers identifiable
|
Detailed home and/or work environment assessment such as pets, occupation
|
|
Coexistent conditions
|
Asthma, eczema (presence of other atopic conditions makes allergic rhinitis more likely)
|
|
Treatments currently using/ previously tried and perceived efficacy – check appropriate use
|
Antihistamines
Intranasal corticosteroid sprays
Combined intranasal corticosteroid and antihistamine sprays
Decongestants
Saline treatments
Other
|
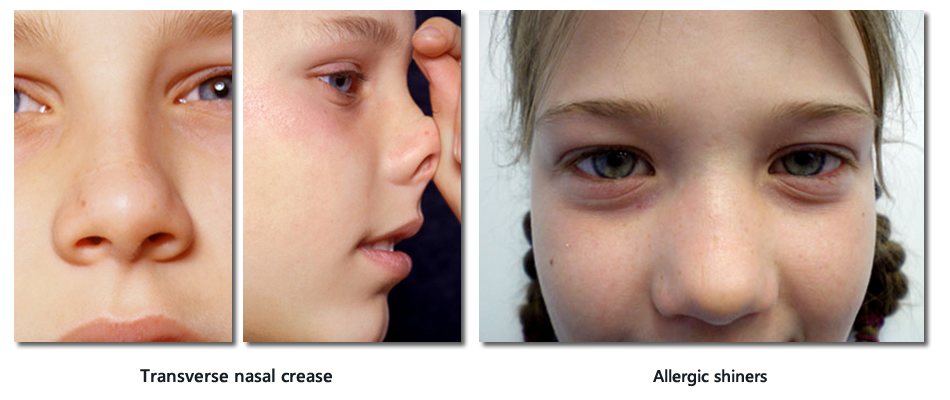
Important signs of allergic rhinitis on physical examination of the face
- Transverse nasal crease, known as the allergic salute from upward rubbing of nose.
- Darkened circles around eyes known as allergic shiners.
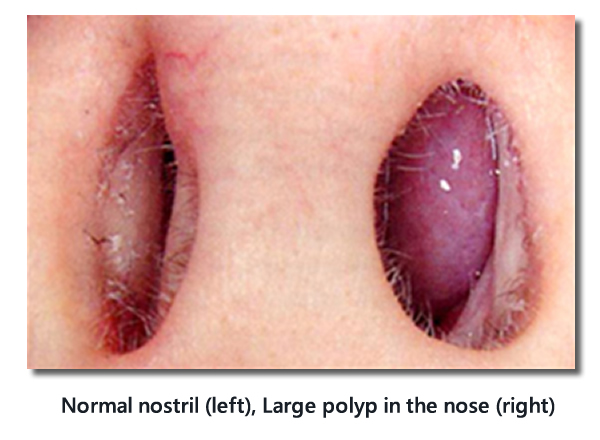
Important signs of allergic rhinitis on physical examination of the nose
Each nostril should be examined with an otoscope.
- Pale, swollen inferior turbinate(s)
- +/- Strands of mucus
- +/- Clear watery discharge
- +/- Exclude presence of large polyps
Normal nostril (left), Large polyp in the nose (right)
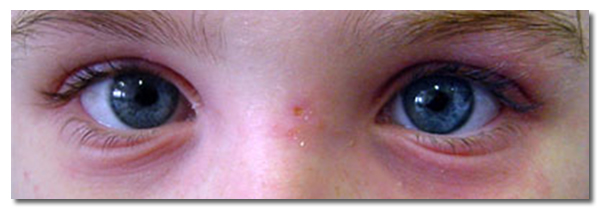
Important signs of allergic rhinitis on physical examination of the eyes
- Red, oedematous eyelids
- Conjunctiva papillae
Allergy testing
Pharmacotherapy to treat allergic rhinitis can be initiated without waiting for diagnostic allergy testing. Testing increases the accuracy of diagnosis and identification of potential aeroallergen triggers.
Diagnostic allergy testing involves either:
- Skin prick testing (SPT) or
- Serum allergen specific immunoglobulin E (ssIgE) testing
These tests detect the presence of IgE antibodies to allergens and their possible clinical relevance.
Procedures for allergy testing
SPT involves pricking the person's skin with commercially available aeroallergen/s. After 15-20 minutes, positive reactions are read, and the wheal size recorded. Patients should avoid antihistamines and drugs with antihistamine activity such as pizotifen and tricyclics for 3-4 days prior to SPT.
Serum specific IgE tests to aeroallergens are blood tests which are available for dust mite, pollen mixes, mould mixes and animal dander. It is important to note that only certain aeroallergen/s in mixes may be clinically relevant. Antihistamines do not affect the results of ssIgE testing.
Limitations of allergy testing for aeroallergen sensitisation
Test results must be interpreted by clinicians experienced in performing and interpreting these tests, in conjunction with the patient's clinical history.
Positive SPT or ssIgE test results do not automatically prove the allergen/s are causing the symptoms. They only confirm the presence of IgE antibodies or sensitisation to that allergen:
- Positive SPT or ssIgE test results to a particular aeroallergen/s may not be clinically relevant. In a patient with seasonal symptoms but positive SPT or ssIgE test results to dust mite, for example, dust mite is unlikely to be clinically important since they are present all year round.
- SPT wheal size or ssIgE level to aeroallergens cannot be used to determine the clinical significance of the trigger. Severe dust mite interpretation cannot be taken to imply the patient has a clinically severe dust mite allergy.
- Knowledge of common inhalant allergens relevant to the geographical location of the patient is required to ensure that tests are initiated for the relevant aeroallergens.
Food specific IgE testing should not be performed in allergic rhinitis investigation because:
- Food allergy is not a cause of intermittent or persistent allergic rhinitis. Acute onset rhinitis with symptom resolution typically occurs within 24 hours as part of an IgE mediated food allergic reaction.
- Irrelevant positive results may occur, and these may cause unnecessary concern.
A full blood count and total IgE is of little clinical use in the investigation of allergic rhinitis.
Non evidence-based methods that claim to test for allergy
Non evidence-based testing methods include IgG testing, cytotoxic food testing, kinesiology, Vega testing, electrodermal testing, pulse testing, reflexology and hair analysis. These unproven tests are not scientifically validated and may lead to unnecessary and costly avoidance strategies.
They are not Medicare rebated in Australia or Pharmac rebated in New Zealand. These methods are not recommended by ASCIA or the World Allergy Organisation (WAO). Further information www.allergy.org.au/patients/allergy-testing
Differential Diagnosis
Non-allergic and allergic rhinitis can co-exist in the same patient. Non-allergic rhinitis encompasses a range of disorders where rhinitis (nasal obstruction and/or rhinorrhea) is not caused by IgE mediated aeroallergen allergy.
|
Differentials to consider |
Key features |
|
Chronic rhinosinusitis/polyposis
|
Anosmia, facial pressure/pain, muco-purulent discharge
|
|
Non-allergic rhinitis with eosinophilia
|
Negative allergy tests but > 20% eosinophils on nasal smear
|
|
Hormonal |
Pregnancy
Menstrual cycle rhinitis
|
|
Drug induced |
Typically, aspirin and other NSAIDs. Range of other medications also reported include decongestants, ACE inhibitors, alpha-adrenoceptor antagonists, oral contraceptive pill, chlorpromazine, and methyldopa
|
|
Granulomatous diseases
|
External nasal swelling, sinusitis, nose bleeds, septal perforation, collapse of nasal bridge, multi-system involvement
|
|
Idiopathic/vasomotor rhinitis |
Sudden onset and offset of watery nasal discharge.
Can be triggered by strong smells or changes in environmental temperature
|
When to consider other conditions
|
Feature |
What to consider |
|
Unilateral nasal obstruction
|
Foreign body in children, nasal polyp, deviated septum, tumor
|
Discharge
|
Chronic rhinosinusitis or super-imposed infection
Foreign body (children), CSF leakage
|
|
Negative allergy tests |
Correct aeroallergens selected.
Non-allergic rhinitis
|
|
Failure to respond to allergic rhinitis therapy |
Compliance
Non-allergic rhinitis
|
Referral to a specialist
Referral to a clinical immunology/allergy specialist should be considered if:
- Further allergy testing and interpretation is required to confirm diagnosis and facilitate allergen avoidance.
- Allergic rhinitis is severe or inadequately controlled despite therapy.
- Consideration if being made for allergen immunotherapy.
- Other atopic comorbidities require management.
Referral to an ENT surgeon should be considered if there is medically refractory nasal obstruction.
Aeroallergen Minimisation
- Avoidance or minimisation of confirmed allergen/s may assist some people in reducing the severity of their allergic rhinitis symptoms.
- This can be difficult to achieve for house dust mite and pollens.
- Avoidance strategies must only be developed if the allergens are clinically significant.
- Realistic consideration must also be given to the family’s ability to action strategies.
House dust mites
- House dust mites are microscopic arthropods that live indoors and feed on human skin flakes.
- Two major species are Dermatophagoides pteronyssinus (most common) and Dermatophagoides farinae.
- They thrive in temperate and humid climates.
- The major allergen excreted by house dust mites are the digestive enzymes in their faeces.
- Life span of a house dust mite is approximately two months. In this time each house dust mite can produce 2,000 faecal particles.
- House dust mite minimisation is possible, but eradication is not.
House dust mite minimisation
- Advice regarding bedding:
- Wash weekly in hot water (> 60oC).
- If washing in cold water, use product containing tea tree oil.
- Hot tumble dry washed items for 10 minutes to kill dust mite.
- Use dust mite impermeable covers on pillows and mattresses.
- Remove sheepskins and woollen underlays.
- Wash soft toys in eucalyptus oil or place in freezer overnight.
- Vacuum carpets weekly.
- Damp dust or use electrostatic cloths for hard surfaces weekly.
Pollen
Pollen that causes allergic rhinitis are usually:
- Wind pollinated grasses, weeds, and trees.
- Not caused by Australian or New Zealand native plants.
- Not caused by highly flowered plants as they produce less pollen which is transported by bees, other insects, or birds.
Recommended actions for patients to reduce exposure to pollen:
- Remain indoors during, before or after thunderstorms, and on windy days. When in contact with water, pollens release starch granules which can trigger allergic rhinitis and asthma symptoms, known as thunderstorm asthma.
- Avoid activities known to cause allergen exposure such as mowing grass.
- Shower after outdoor activities where exposure to pollen is high.
- Use re-circulated air in car when pollen levels are high.
- Wear sunglasses to reduce the amount of pollen that gets into eyes.
- Dry bedding and clothing inside or in a tumble dryer.
Pet dander
- Domestic pets can be a major source of allergens in the home.
- Any animal with fur can be a source of pet allergy, but pet allergies are most commonly associated with cats and dogs.
- Allergens are found in skin cells the animal sheds (dander), saliva, urine and sweat.
- Pet saliva can stick to carpets, bedding, furniture, and clothing. Allergens can become airborne for prolonged periods.
- Clear demonstration of pet dander triggering symptoms needs to occur before recommending removing pet.
- The amount of fur shed can vary between breeds, but no breed is truly hypoallergenic.
Pet dander minimisation
- Discuss removing the pet from the home if symptoms are severe. It can take an average of 20 weeks before cat allergen concentration reaches comparable levels to a house without a cat.
- If dander is only causing minor problems, consider keeping the pet outside.
- The effectiveness of washing pets regularly and the use of HEPA air filters is uncertain.
Mould
- Exposure to mould can occur both indoors and outdoors even in dry climates.
- Mould in the home can typically be found in damp, warm and poorly lighted areas. It causes discoloration of surfaces and/or a musty smell.
- Outdoor moulds can be present in all conditions, particularly in humid climates, with seasonal peaks.
Mould avoidance
Recommended actions for patients:
- Remove visible mould by cleaning with diluted bleach, vinegar, or other mould reduction cleaners.
- Ensure adequate ventilation.
- Dry or remove wet carpet.
- Fix any leaks.
- Remove indoor pot plants.
- Do not mow lawns or work with garden compost and mulch.
Medications for Allergic Rhinitis
The duration and severity of allergic rhinitis symptoms are useful in guiding therapy, as shown in the table below.
Definitions
- Intermittent: <4 days/week or <4 weeks.
- Persistent: >4 days/week or >4 weeks.
- Mild: Normal sleep, no impairment of daily activities, normal work, or school performance.
- Moderate-severe: One or more of the following: abnormal sleep, impairment of activities, abnormal work or school performance.
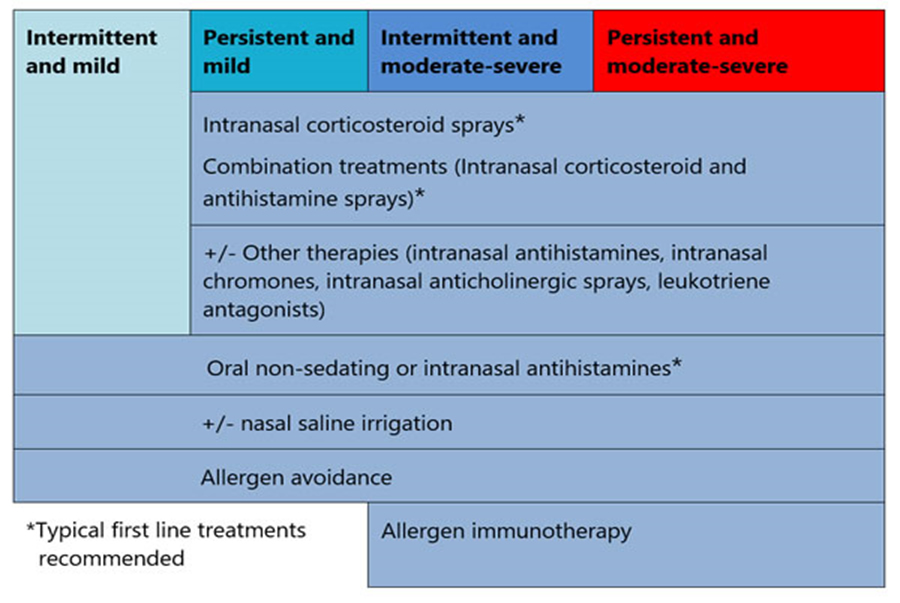
Allergic rhinitis pharmacotherapy options
|
First line treatment options
|
Other possible treatments
|
Short term treatment options
|
|
Antihistamines (non-sedating oral or intranasal)
|
Saline treatments
|
Decongestants (oral or intranasal)
|
|
Intranasal corticosteroid sprays
|
Intranasal chromones
|
Systemic oral corticosteroids
|
|
Combined intranasal corticosteroid and antihistamine sprays*
|
Intranasal anticholinergic sprays
|
Combined intranasal decongestant and antihistamine sprays*
|
|
*Some require a prescription
|
Oral leukotriene antagonists
|
*Some require a prescription
|
Allergic rhinitis pharmacotherapy principles
- When symptoms improve, pharmacotherapy doses may be reduced.
- Trial of pharmacotherapy initiated by primary care physicians and maintained for at least 4 weeks is recommended. Then consider referral to a specialist if no improvement.
- If a patient is a competitive athlete, it is important to ensure medications suggested are permitted. For example, pseudoephedrine used in some decongestants is subject to certain restrictions. Contact Sport Integrity Australia or Drug Free Sport New Zealand for information.
Non-sedating antihistamines
|
Place in therapy
|
First line treatment for intermittent mild allergic rhinitis or used in conjunction with other therapies
|
|
Route
|
Rapid onset action (1-2 hours)
Very rapid onset action (within 30 minutes). May be used as a rescue medication to provide immediate relief of symptoms
|
|
Availability
|
Over the counter
|
|
Type
|
Non-sedating antihistamines are recommended
Sedating antihistamines are not recommended
|
|
Frequency of use
|
Once or twice a day
|
|
Benefits
|
↓ itchy, watery eyes
↓ sneezing, itchy, runny nose
Limited decrease in symptoms
|
Whilst some nasal antihistamines can reduce nasal congestion, intranasal corticosteroids (INCS) are more effective in reducing nasal congestion.
Intranasal corticosteroids (INCS)
|
Place in therapy
|
First line treatment for persistent and/or moderate to severe allergic rhinitis and treatment failures with antihistamines alone.
|
|
Availability
|
Over the counter or prescribed by a doctor
|
|
Age restriction
|
Different intranasal corticosteroids often have different minimum age restrictions.
|
|
Frequency of use
|
Continuous use is needed for long term benefit.
Long term use is recommended if effective.
Use on an as-needed basis is less effective.
|
|
Benefits
|
↓ itchy, watery eyes
↓ sneezing, itching, runny nose
↓ nasal congestion
|
Note:
- Different brands of INCS vary in strength and efficacy.
- Combined intranasal corticosteroid and antihistamine sprays offer the advantages of both medications.
Side effects of intranasal corticosteroids (INCS)
- Local side effects (uncommon when correctly administered) include:
- Dryness
- Epistaxis (occasionally)
- Topical corticosteroids such as INCS do not cause atrophy.
- Minimal potential for systemic absorption, when used in recommended doses.
- Nasal irritation may occur, but this is uncommon.
Whilst systemic absorption of INCS is negligible, growth of children and adolescents taking corticosteroids by any route should be monitored
INCS must be used with caution in patients with pre-existing glaucoma and/or cataracts. Rare cases of cataracts, glaucoma and increased intraocular pressure have been reported following use of INCS.
Correct administration of INCS

Patients should be instructed on the correct and consistent use of prescribed treatment and given an ASCIA Treatment Plan for Allergic Rhinitis.
Saline nasal irrigation
- Is usually well tolerated, effective in reducing rhinitis symptoms, safe and inexpensive.
- Clears aeroallergens and inflammatory mucus but does not replace pharmacotherapy.
- Large volume (>60 mL) and positive pressure devices are more effective than simple sprays (<1 mL).
Intranasal ipratropium
- Anticholinergic sprays may be used for non-allergic rhinitis.
- Only decreases watery rhinorrhoea.
- May be used in allergic rhinitis as adjunct treatment for persistent rhinorrhoea, with use of antihistamines or intranasal corticosteroid.
Oral leukotriene antagonists
- Used in children/adolescents with asthma and allergic rhinitis.
- No additional benefit if used in combination with antihistamines.
- The combination of leukotriene antagonists (such as montelukast) and antihistamines are no more effective than intranasal corticosteroids alone.
- There is no Australian or New Zealand government subsidy for use of leukotriene antagonists for patients with allergic rhinitis alone.
Decongestants
- Oral or nasal decongestants may be used short term (3-5 days) to reduce nasal congestion if severe. This allows more effective administration of intranasal corticosteroids if turbinates are very swollen.
- Chronic use of intranasal decongestants may lead to rebound nasal obstruction, called rhinitis medicamentosa.
- Decongestants should not be used in patients with hypertension, coronary artery disease, prostatism or glaucoma. They should not be used in pregnancy.
Systemic steroids
- Brief courses of oral corticosteroids (3-7 days) are rarely indicated, but may be considered:
- If there is severe nasal obstruction.
- As short-term rescue medication if symptoms are severe, despite conventional therapy, but only up to a maximum limit of 2 or 3 short courses in a 12-month period.
- Depocorticosteroids are not recommended due to short duration of benefit and potential for local (subdermal and dermal atrophy) and systemic side effects.
- Patients requiring oral corticosteroids for allergic rhinitis should be referred to a clinical immunology/allergy specialist for assessment.
Ocular treatment
- Non-pharmacological therapy:
- Flush allergen from eyes (saline washes, liquid-tear preparations).
- Cool compresses.
- Oral antihistamines or topical mast cell stabilisers may be used to control itchy/watery eyes.
- Intranasal corticosteroids can reduce ocular symptoms of allergic rhinitis.
- Ocular corticosteroids should only be prescribed in consultation with, and regular review by an ophthalmologist.
Chronic Rhinosinusitis with Nasal Polyps (CRSwNP) treatment
- Nasal irrigation is a widespread first line treatment.
- Intranasal corticosteroids are safe and effective for long term use.
- If short courses of oral corticosteroids are prescribed, both patients and practitioners must remain vigilant to avoid side effects.
- A short course of antibiotics may have a role to play in non-Type 2 CRSwNP, but patient selection is important, and side effects need to be managed.
- The effectiveness of allergen immunotherapy in the treatment of CRSwNP remains unclear.
- Biologics can have positive outcomes and may be usede when other treatments are not effective.
- Endoscopic sinus surgery plays a significant role in the management of CRSwNP.
- Surgery is safe, reduces symptom burden, and improves quality of life.
- Refer to an ENT or clinical immunology/allergy specialist for treatment.
For further information, a treatment algorithm is available in the ASCIA CRSwNP Position Paper
Surgery
- Surgery plays a limited role in the management of rhinitis, except for CRSwNP.
- Turbinate reduction and re-modelling of the nasal airway can improve medically refractory nasal obstruction.
- Vidian neurectomy (division of autonomic nasal nerves) is not indicated for allergic rhinitis but can be considered for severe intractable watery rhinorrhoea of non-allergic rhinitis (vasomotor rhinitis).
Allergen Immunotherapy for Allergic Rhinitis
Allergen immunotherapy, also known as desensitisation:
- Involves the regular administration of commercially available allergen preparations to promote clinical tolerance to the allergen/s, administered by subcutaneous injections or sublingual preparations.
- Is usually administered for 3-5 years to produce durable effects, to reduce the frequency and severity of allergic rhinitis symptoms.
- Should only be initiated by medical specialists with training in allergy, following a confirmed diagnosis.
Benefits
- Decreases severity of allergic rhinitis symptoms and the need for medications.
- Individual patients will experience different degrees of benefit, and on average there may be a 50% reduction in symptoms and/or medication need.
Possible additional benefits include:
- Reduced risk of new sensitisation (developing IgE antibodies) from few to multiple aeroallergens in children.
- Reduced risk of progression from allergic rhinitis to asthma in children.
- Reduced asthma exacerbation.
Commercial aeroallergens available for allergen immunotherapy in Australia and New Zealand include:
- House dust mite
- Pollens (grass, tree, and weeds)
- Animal dander
- Moulds
Referral to a specialist
Consider referring a patient to a clinical immunology/allergy specialist for allergen immunotherapy when:
- Allergic rhinitis is causing severe and/or persistent symptoms.
- Medications:
- Are associated with intolerable side effects.
- Do not adequately control symptoms.
- May be effective, but patient desires to reduce use.
- Allergen avoidance is difficult, as in the case of pollen.
- A patient has an occupational allergy such as a veterinarian with animal dander allergy.
For more information refer to ASCIA allergen immunotherapy e-training for health professionals
© ASCIA 2024
Content updated 2024
For more information go to www.allergy.org.au/hp/allergic-rhinitis
To support allergy and immunology research go to www.allergyimmunology.org.au/donate



