Anaphylaxis Checklists
ASCIA has developed the following five checklists to assist in the management of patients who are at risk of severe allergic reactions (anaphylaxis).
Anaphylaxis Checklist - Prescribers (Doctors and Nurse Practitioners)
Anaphylaxis Checklist - Pharmacists
Anaphylaxis Checklist - Patients and Carers
Anaphylaxis Checklist - Prescribers
![]() ASCIA HP Checklist Anaphylaxis Prescribers 2025121.28 KB
ASCIA HP Checklist Anaphylaxis Prescribers 2025121.28 KB
 This document has been developed by ASCIA, the peak professional body of clinical immunology/allergy specialists in Australia and New Zealand. ASCIA information is based on published literature and expert review, is not influenced by commercial organisations and is not intended to replace medical advice. For patient or carer support contact Allergy & Anaphylaxis Australia or Allergy New Zealand.
This document has been developed by ASCIA, the peak professional body of clinical immunology/allergy specialists in Australia and New Zealand. ASCIA information is based on published literature and expert review, is not influenced by commercial organisations and is not intended to replace medical advice. For patient or carer support contact Allergy & Anaphylaxis Australia or Allergy New Zealand.
The aim of this checklist is to assist doctors and nurse practitioners who prescribe adrenaline (epinephrine) devices for patients who are at risk of having a severe allergic reaction (anaphylaxis).
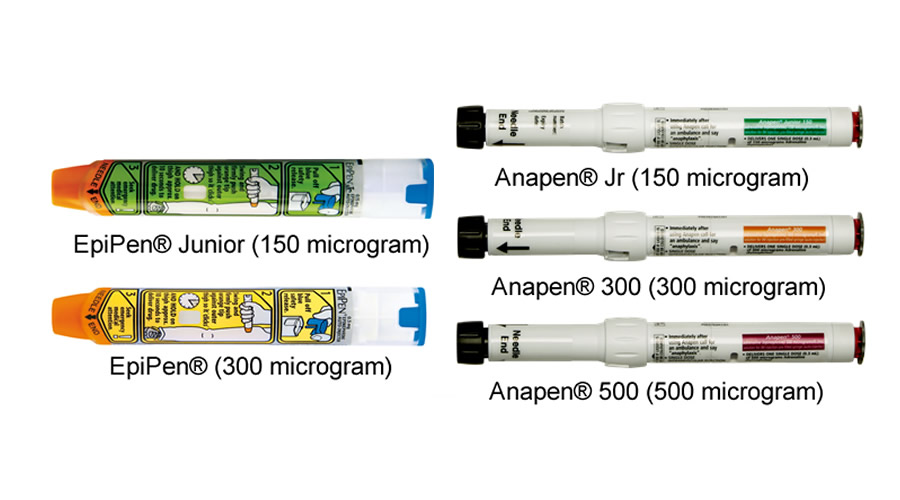
There are two brands of adrenaline devices, EpiPen® and Anapen®:
- EpiPen® (300 mcg) and EpiPen® Jr (150 mcg) are rebated by the PBS in Australia and Pharmac in New Zealand.
- Anapen® 500 is rebated by the PBS in Australia.
Adrenaline devices are prescribed as follows:
- 150 mcg devices for children weighing 7.5 to 20kg.
- 300 mcg devices for children weighing over 20kg and adults, including pregnant women.
- 500 mcg or 300 mcg devices for children weighing over 50kg and adults, including pregnant women.
- Record history of the allergic reaction and suspected triggers using the ASCIA event record form
- Prescribe adrenaline device and check that the dose is appropriate (see above). A specialist referral is not required for dose adjustment.
- Check that the prescription is for two devices, which are rebated by the PBS in Australia and Pharmac in New Zealand.
- For newly diagnosed patients contact a specialist (allergy, respiratory, paediatrician), if necessary, for authority prescription, pending specialist appointment and inform patient/carer about support organisations, Allergy & Anaphylaxis Australia or Allergy New Zealand
- Ensure that the patient has been referred to a clinical immunology/allergy specialist, who is listed on the ASCIA website. allergy.org.au/patients/locate-a-specialist
- Complete and sign ASCIA Action Plan for Anaphylaxis (RED) - the latest version is on the ASCIA website
- Complete ASCIA Travel Plan if required.
- Inform patient that they should always carry their adrenaline device/s and ASCIA Action Plan.
- Ensure the patient/carer understands that that adrenaline is the first line treatment for anaphylaxis and that antihistamines should not be used for the treatment for anaphylaxis. If antihistamines are used to treat mild to moderate allergic reactions, only non-sedating antihistamines should be used.
- Educate patient/carer on how to give the adrenaline device (using trainer devices), recognition and treatment of allergic reactions, carrying and storage of the adrenaline device and appropriate allergen avoidance measures. For information go to allergy.org.au/anaphylaxis
- If patient has asthma, review and optimise asthma management and educate about asthma and anaphylaxis. allergy.org.au/patients/asthma-and-allergy/asthma-and-anaphylaxis
- Inform patient/carer to check and note the expiry date of their device/s.
- Encourage an appointment every 12 to 18 months, to prescribe new adrenaline device/s before they expire, to review if new allergies have developed or more severe allergic reactions have occurred, and to renew the patient’s ASCIA Action Plan for Anaphylaxis.
For adrenaline device supply updates check www.allergy.org.au/members/adrenaline-device-availability
© ASCIA 2025
Content updated March 2025
For more information go to www.allergy.org.au/hp/anaphylaxis
To support allergy and immunology research go to www.allergyimmunology.org.au/donate