ASCIA Action Plans and First Aid Plans for Anaphylaxis
ASCIA Action Plans for Anaphylaxis (RED) | ASCIA Travel Plan | ASCIA Action Plan for Allergic Reactions (GREEN) | ASCIA Action Plan for Drug (Medication) Allergy (GREEN) | ASCIA First Aid Plan for Anaphylaxis (ORANGE) | ASCIA First Aid Plan for Anaphylaxis (ORANGE) Pictorial Poster | Important Information | How to Order Hard Copies of ASCIA Action Plans
ASCIA Action Plans for Anaphylaxis are emergency response plans for severe allergic reactions (anaphylaxis):
- These plans are medical documents that are completed and signed by the treating doctor or nurse practitioner.
- The treating doctor or nurse practitioner can type in patient details, insert a digital photograph and signature - first save the plan as a PDF after typing patient details into the text fields. Alternatively, a physical photograph can be glued to the plan, with a wet or digital signature.
- ASCIA Action Plans do not need to be stamped - a signature is sufficient.
- ASCIA Action Plans do not expire, and therefore the plan is still valid beyond the recommended review date, which is a guide for patients to see their doctor or nurse practitioner.
- Major updates were made in the 2023 versions, and only minor updates have been made to the 2025 versions. Information about what has been updated in the 2025 plans is available here.
- Prior versions (2023) are still be valid for use in 2025.
- Where possible it is preferable to provide ASCIA plans in colour, but black and white plans are valid.
- More information about ASCIA Action Plans is available here.
![]() ASCIA PC Action Plans Anaphylaxis 2025 General Information226.63 KB
ASCIA PC Action Plans Anaphylaxis 2025 General Information226.63 KB
ASCIA Action Plans for Anaphylaxis
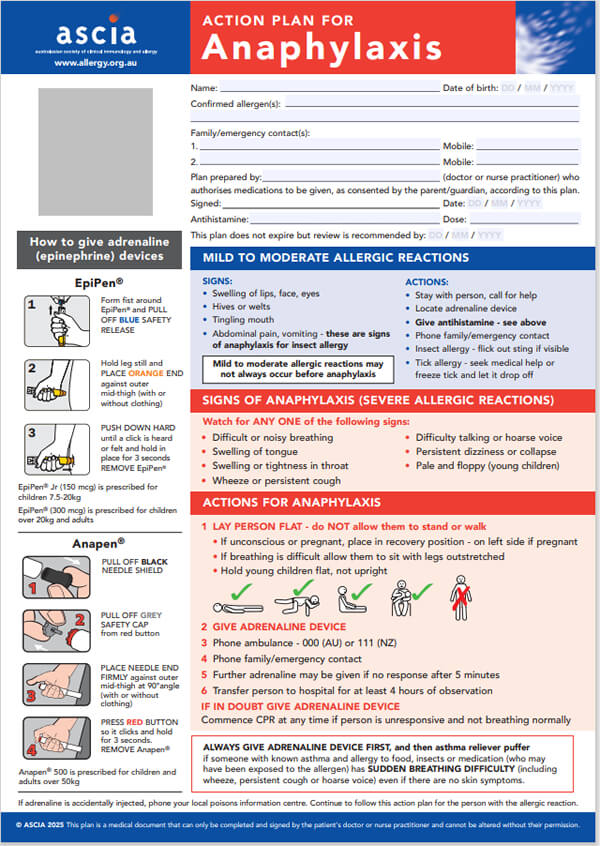
ASCIA Action Plan for Anaphylaxis (RED) 2025The general version of this plan is for people with allergies who have been prescribed either brand of adrenaline (epinephrine) device. Device specific versions are available below. These plans are not for allergic rhinitis (hay fever) due to pollen, dust mite, animals or other inhaled allergens. |
|
 |
 |
|
|
|
|
ASCIA Travel Plan for people at risk of anaphylaxisThis plan is for use with the ASCIA Action Plan for Anaphylaxis
|
|

ASCIA Action Plan for Allergic Reactions (GREEN) 2025This plan is provided to people with allergies who have not been prescribed an adrenaline device. This plan is not for allergic rhinitis (hay fever) due to pollen, dust mite, animals or other inhaled allergens.
|
|

ASCIA Action Plan for Drug (Medication) Allergy (GREEN) 2025This plan is provided to people with drug (medication) allergies who are not usually prescribed an adrenaline device, because accidental exposure to drugs can mostly be avoided. They are usually recommended to wear medical identification, to avoid exposure to drugs used in medical procedures. |
|

ASCIA Record for Drug (Medication) Allergy (GREEN) 2023This record should be used in conjunction with the patient’s ASCIA Action Plan for Drug (Medication) Allergy. |
|
ASCIA First Aid Plans for Anaphylaxis (ORANGE)
This plan can be used as a poster or stored with general use adrenaline devices.
A version of this plan for use by crew on aircraft is available upon request.
For translated versions of the ASCIA First Aid for Anaphylaxis go to www.allergy.org.au/hp/anaphylaxis#ta5
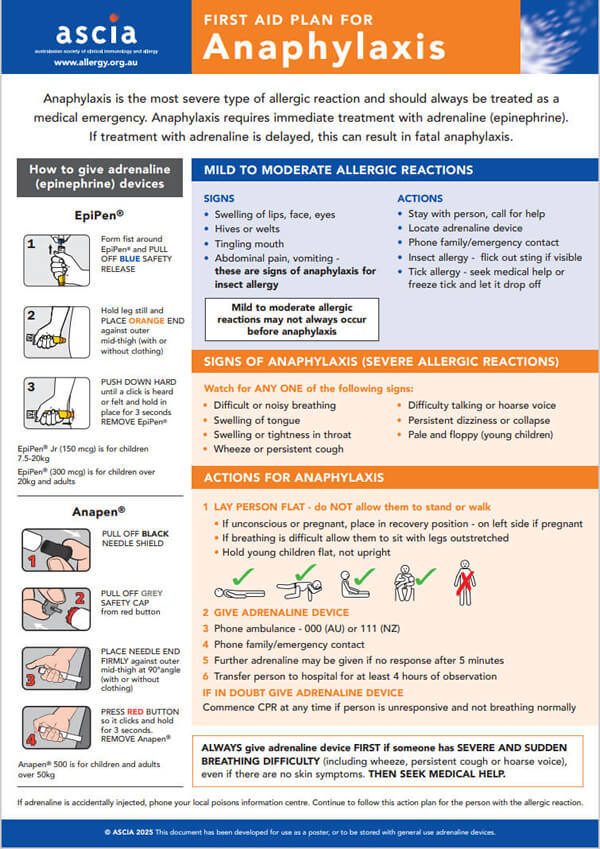
ASCIA First Aid Plan for Anaphylaxis (ORANGE) 2025The general version of this plan can be used as a poster or stored with either brand of general use adrenaline devices. Device specific versions are available below. |
|
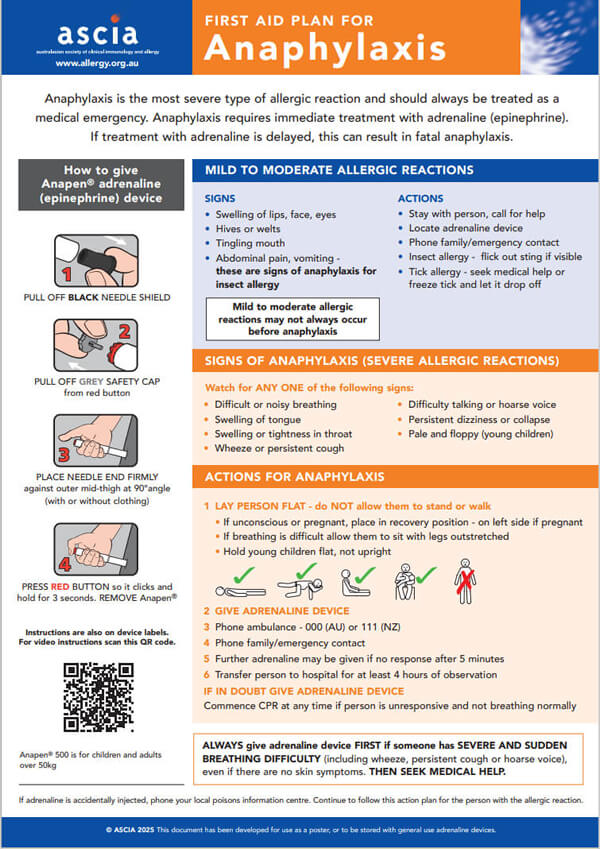 |
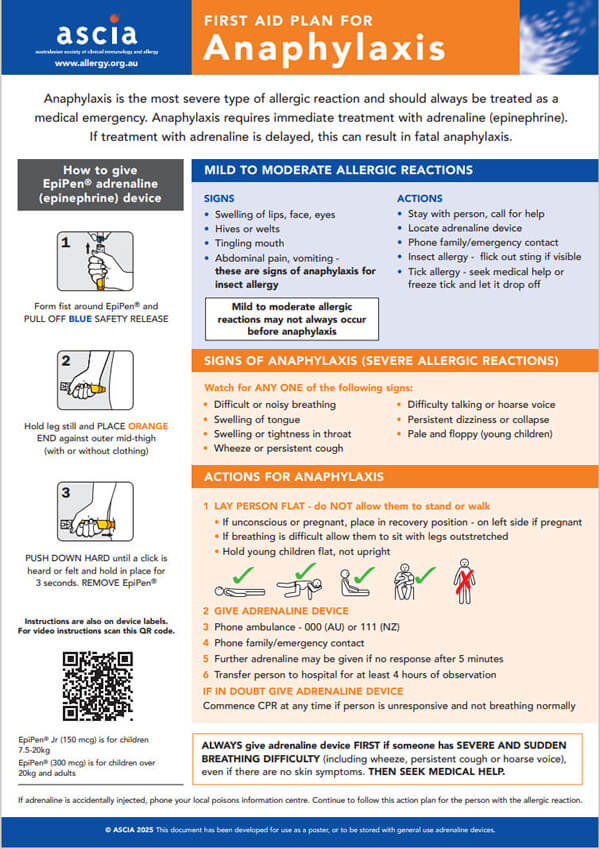 |
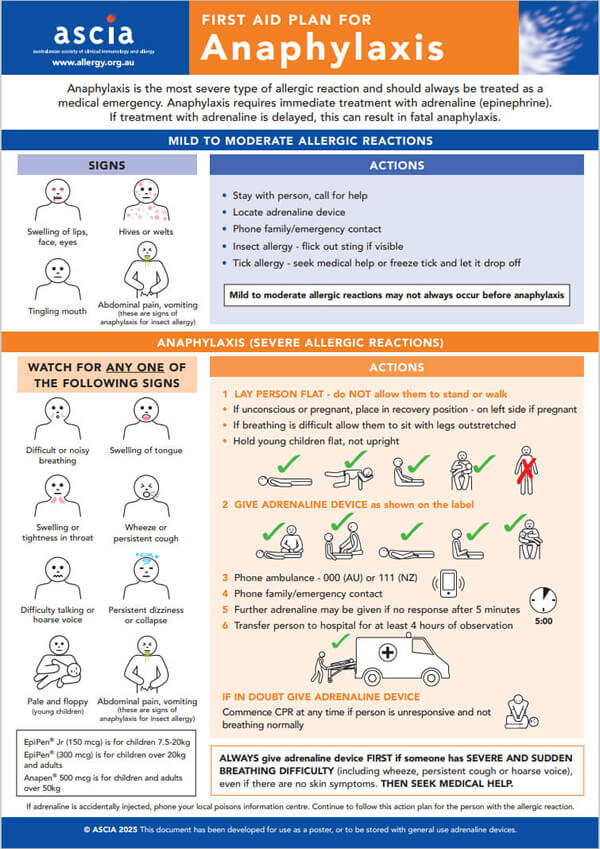
ASCIA First Aid Plan for Anaphylaxis Pictorial Poster (ORANGE) 2025This plan is best viewed when printed as an A3 poster. |
|
Important Information:
-
ASCIA Action Plans were first developed in 2003 to standardise the recognition of signs and actions for mild to moderate and severe allergic reactions (anaphylaxis). This includes easy to follow steps for the emergency treatment of anaphylaxis. ASCIA Action Plans continue to be used as the standard action plans throughout Australia and New Zealand. The content is regularly reviewed by experts and updated as required.
-
ASCIA Action Plans do not expire, but they should be reviewed and updated when patients are reassessed by their doctor or nurse practitioner. This should occur each time they obtain a new adrenaline device prescription, which is approximately every 12 to 18 months. If the patient is a child, the photo should be updated each time, so they can be easily identified.
-
ASCIA Action Plans for Anaphylaxis can be used for people with food, insect, tick and drug allergies, or any other allergy that can result in a severe allergic reaction (anaphylaxis). If a person only has a drug allergy and does not have a prescribed adrenaline device, the ASCIA Action Plan for Drug (Medication) Allergy should be used. These plans are not for allergic rhinitis (hay fever) due to pollen, dust mite, animals or other inhaled allergens.
-
Adrenaline injector devices contain a single, fixed dose of adrenaline and are designed to be used by anyone (medical training is not required), or the patient themselves, if they are not too unwell or too young. It is generally recommended that people who have an adrenaline device should also wear a medical identification/emergency response emblem, or other medical identification.
-
When writing antihistamines on a child’s ASCIA Action Plan, doctors or nurse practitioners should avoid brand names, but instead write the generic name (e.g. Loratadine) and dose (or "as per package") on the plan. Some Children's Education/Care sector regulations state that only the brand name on the plan is allowed (not generic brands). Therefore, using generic names allows flexibility if a brand is unavailable and a generic version is provided.
-
ASCIA Action Plans should be used as part of a comprehensive anaphylaxis management plan that includes:
- Age appropriate education of people with allergies and their peers or colleagues.
- Training in the recognition and management of allergic reactions.
- Development of strategies to reduce the risk of accidental exposure
- An ASCIA Action Plan to be used as an emergency response plan for when exposure to allergens occurs. -
All ASCIA Action, First Aid, Management, Transfer, Travel and Treatment Plans are available free of charge on the ASCIA website as PDFs that can be completed online, downloaded and printed at www.allergy.org.au/hp/ascia-plans-action-and-treatment
How to Order Hard Copies of ASCIA Action Plans
Health professionals can order hard copies (in full colour) of the red ASCIA Action Plans for Anaphylaxis (Anapen version), by submitting an order online using the HCP shop on the Anapen website – anapen.com.au/shop. Password – arrotex. Alternatively, contact Arrotex Customer Service using the contact page – www.anapen.com.au/contact or call 1800 761 964.
Health professionals can order hard copies (in full colour) of red ASCIA Action Plans for Anaphylaxis (EpiPen version), green ASCIA Action Plans for Allergic Reactions and orange ASCIA First Aid Plan for Anaphylaxis (EpiPen version) by emailing Viatris Customer Service (distributor of EpiPen in Australia) - email
For further information about ASCIA Action Plans and other anaphylaxis resources go to www.allergy.org.au/anaphylaxis
For Asthma Action Plans please go to the National Asthma Council Australia website Asthma Action Plans
Content updated February 2025

