Allergen Immunotherapy FAQ - Availability and Regulation
Availability and Regulation of Allergen Immunotherapy (AIT) in Australia - Frequently Asked Questions (FAQ)
Q1. Who supplies AIT?
All suppliers of AIT and skin prick test (SPT) products in Australia and New Zealand are listed on the ASCIA website: Suppliers of AIT ; Suppliers of SPT
Q2. Which AIT products in Australia are TGA-approved?
All AIT products with TGA-approval are entered on the Australian Register of Therapeutic Goods (ARTG): https://www.tga.gov.au/australian-register-therapeutic-goods. The ARTG is a register of medicines that can be lawfully used in Australia.
TGA-approved sublingual (SLIT) tablet and injection (SCIT) treatments are available to treat patients as young as 5 years with allergies to both house dust mite (HDM) and grass pollen (Table 1). HDM and grass pollen allergies are the two most common causes of allergic respiratory disease in Australia.
For individual patients with less common allergies (e.g. to trees, moulds and animals), special exemptions apply to enable the use of suitable AIT products without an ARTG entry.
Note: It is often not possible to access TGA approved allergen immunotherapy due to product supply issues, the low number of TGA approved products and a TGA approval process that is not feasible for a wide range of immunotherapy products.
Table 1. TGA-approved AIT products currently produced for supply to Australia.
Dose = recommended / maximum recommended maintenance dose.
|
Allergen class |
Registration name |
Formulation |
Allergen profile |
Dose |
Approved age indication |
|
HDM |
ALUSTAL® House dust mite extract |
SCIT |
D. pter + D. far |
8 IR |
≥ 5 years |
|
ACTAIR® |
SLIT tablet |
D. pter + D. far |
300 IR |
≥ 5 years |
|
|
ACARIZAX® |
SLIT tablet |
D. pter + D. far |
12 SQ-HDM |
≥ 12 years |
|
|
Grass pollen |
ALUSTAL® Pollen extract of five grasses |
SCIT |
Rye grass, Cocksfoot, Sweet vernal grass, Meadow grass and Timothy |
8 IR |
≥ 5 years |
|
ORALAIR® |
SLIT tablet |
Rye grass, Cocksfoot, Sweet vernal grass, Meadow grass and Timothy |
300 IR |
≥ 5 years |
|
|
GRAZAX® |
SLIT tablet |
Timothy |
75,000 SQ-T |
≥ 5 years |
Q3. Which AIT products are ‘Named Patient Products’?
‘Named Patient Products’ are unapproved AIT treatments (i.e. products without an ARTG entry) that are ordered for individual ‘named patients’ under a special exemption. This exemption applies to all prescription (Schedule 4) medicines and is made available to cover circumstances where a TGA-approved medicine is unavailable to treat a patient’s specific condition.1-3
Treatments accessed under this general ‘named patient’ exemption must be ‘dispensed or extemporaneously compounded [prepared]’ on an individual patient basis.1-3 For AIT products imported as finished prescription medicines, an individual ‘named patient’ order for a single order of treatment should be issued directly to the overseas manufacturer.
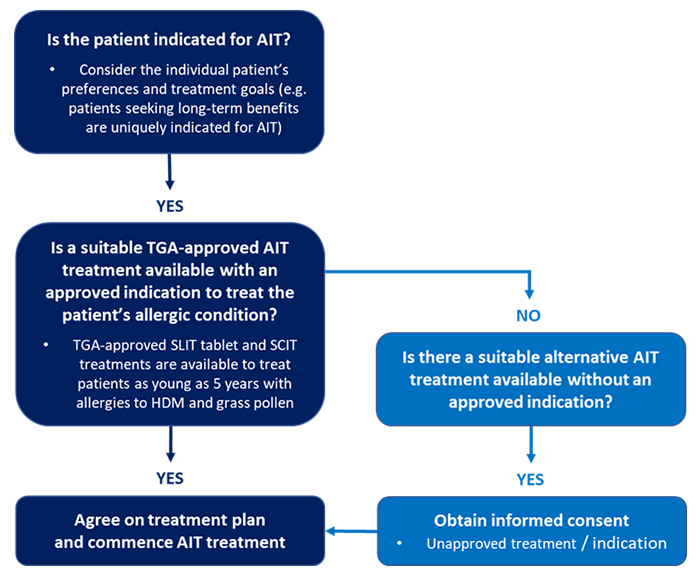
Under this exemption, the same principles and considerations apply for all prescription medicines: based on the patient’s specific condition, individual physicians are responsible for considering the suitability of available TGA-approved products and evaluating whether use of an alternative unapproved AIT product can be clinically justified (Figure 1).1-3
In Australia, there is no special TGA policy/scheme that specifically regulates access to unapproved AIT products as ‘Named Patient Products’.4,5
Figure 1. Considering patient suitability for AIT treatments: General flowchart.
Q4. How can AIT products be accessed under the TGA Special Access Scheme and Authorized Prescriber Scheme?
Specific prescribers, or specific groups of prescribers, can apply for an exemption to use unapproved AIT products under the following pathways:
- The TGA Authorized Prescriber Scheme (Standard Pathway). This pathway requires specific, prior approval from a human research ethics committee or endorsement from a specialist college. No specialist colleges currently provide this endorsement for AIT products, so local ethics approval is required.
- The TGA Special Access Scheme (Category B). This pathway requires prescribing physicians to submit an application to the TGA for each prescription, supported by a written clinical justification. For practical reasons, no AIT products are currently accessed under this pathway.
There is no ‘Established History of Use’ exemption available for AIT products under the TGA Special Access Scheme Category C or Authorized Prescriber Pathway.
In practical terms, unless covered by a local ethics approval (and exemption under the Authorized Prescriber Scheme Standard Pathway), prescribing physicians must access unapproved AIT products under a ‘Named Patient Product’ exemption (see Q2).
Q5. How does the TGA ensure the quality, safety and efficacy of AIT products?
For TGA-approved AIT treatments:
- The TGA grants approval and entry on the ARTG only after stringent evaluation of product-specific evidence for quality, safety and efficacy.
- The TGA fully evaluates the balance between the benefits and the risks for patients before approving the recommended indication.6
- The TGA reviews in detail all information and recommendations in the approved Product Information and patient Consumer Medicine Information.
- The TGA requires ARTG entry holders (sponsors) to meet all pharmacovigilance reporting responsibilities; this enables the TGA to perform ongoing monitoring to evaluate whether the benefits of approved medicines continue to outweigh the risks.
For unapproved AIT products:
- There is no stringent evaluation or ongoing monitoring of product quality, safety and efficacy by the TGA.
- Manufacturers or local distributors have no requirement to demonstrate quality, efficacy or safety, or to provide pharmacovigilance reports to the TGA.
- Advice on treatments (e.g. indications, recommended dosing and patient treatment advice) in documents provided by manufacturers or local distributors are not reviewed by the TGA and may not be independently evaluated or be adequately supported by available evidence.
- Individual physicians (with the co-operation of local ethics committees, in the case of exemptions under the TGA Authorized Prescriber Scheme) have primary responsibility for evaluating whether use of specific unapproved AIT products can be clinically justified, based on available evidence (Figure 1).
References:
- Therapeutic Goods Act 1989 (Cth) s. 3-2
- Therapeutic Goods Regulations 1990 (Cth) sub-reg. 12(1) and Schedule 5(6)
- Pharmacy Board of Australia – Guidelines on compounding medicines (2015; updated August 2017) <https://www.pharmacyboard.gov.au/Codes-Guidelines.aspx>
- Bonertz et al. Challenges in the implementation of EAACI guidelines on allergen immunotherapy: A global perspective on the regulation of allergen products. Allergy. 2018; 73: 64–76)
- ‘Accessing unapproved products’, TGA. <https://www.tga.gov.au/accessing-unapproved-products >
- ‘Product regulation according to risk’, TGA. <https://www.tga.gov.au/product-regulation-according-risk >
Content updated July 2023
