ASCIA-TAPID Consensus Guideline - Diagnosis, Management and Transplantation of SCID in Australia and New Zealand
This Consensus Guideline outlines the recommendations from the ASCIA Transplantation and Primary Immunodeficiency (TAPID) group for the diagnosis, management and transplantation of patients with Severe Combined Immunodeficiency (SCID). It also provides a proposed framework for the early investigation, management and supportive care prior to Haematopoietic Stem Cell Transplantation (HSCT), in the anticipation of introduction of routine newborn screening (NBS) for SCID in Australia.
This Guideline was developed by the ASCIA TAPID Group in collaboration with ANZCHOG, the Australian & New Zealand Children Haematology/Oncology Group.
TAPID membership: www.allergy.org.au/about-ascia/ascia-initiatives/tapid
![]() ASCIA HP Guidelines SCID 2019297.58 KB
ASCIA HP Guidelines SCID 2019297.58 KB
Introduction | Diagnosis of SCID | Definitions of SCID | Supportive care for SCID patients prior to HSCT | Curative treatment for SCID | Use of conditioning | Appendices | References
Introduction
Severe Combined Immunodeficiency (SCID) is a heterogeneous group of disorders characterised by impaired T cell (T lymphocyte) development, and resulting impaired B cell (B lymphocyte) function and antibody (immunoglobulin) production1-3. The majority of patients have a genetically identifiable cause (~90%)2. SCID is typically fatal in the first two years of life without definitive intervention.
In the absence of NBS, patients typically present within the first few months of life with severe, recurrent or persistent and opportunistic infections, particularly of the respiratory and gastrointestinal tract, as well as persistent or extensive oral or napkin candidiasis. They can have enteropathy with failure to thrive, persistent diarrhea following rotavirus vaccine, an eczema-like rash and alopecia1,3,4. SCID patients who have received Bacille Calmette-Guérin (BCG) vaccine at birth are also at risk of disseminated BCG infection4.
The incidence of SCID is estimated at 1:58,000 births5,6 with males affected more often than females in an outbred population due to X-linked inheritance in the most common SCID genotype. There were 311,000 live births in Australia in 20167, and hence six new cases of SCID would be expected around the country every year. There were 60,000 live births in New Zealand in the same time period, and therefore one new case per year would be anticipated.
Diagnosis
Newborn screening (NBS)
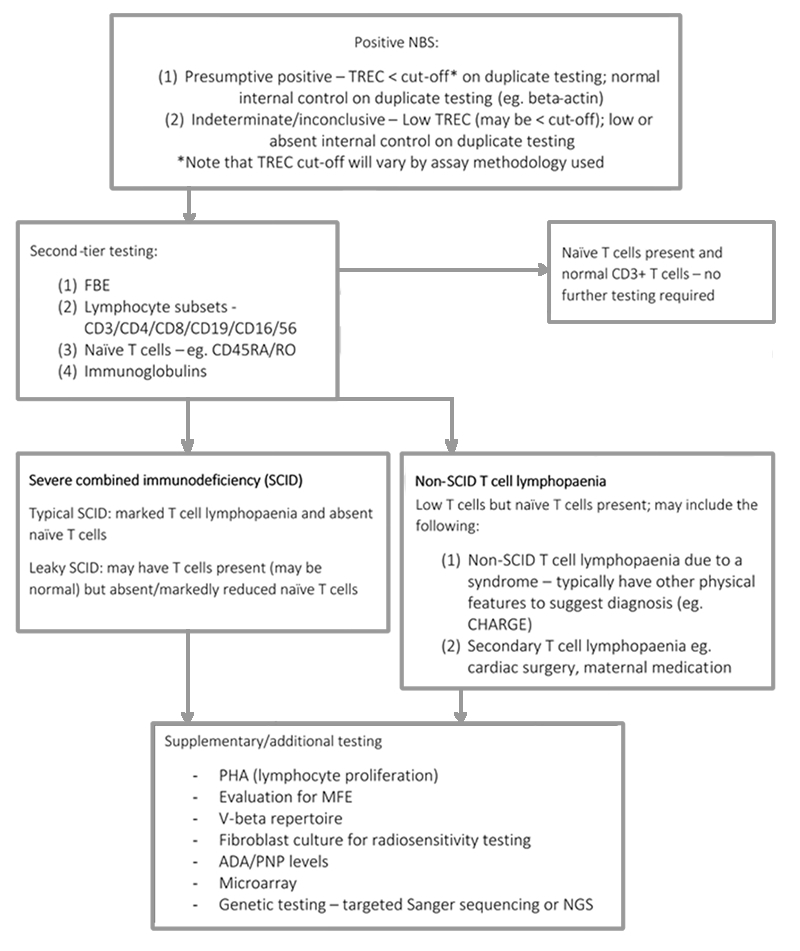
A reliable NBS test is available for SCID, which identifies the presence or absence of adequate levels of T cell receptor excision circles (TREC)8,9. Infants with SCID, irrespective of their genetic aetiology, will have impaired T cell development, and will typically have significantly reduced numbers of thymically derived T cells, and therefore very low or absent TREC10.
Infants with non-SCID lymphopaenia will also be identified by this screening test. The majority of these patients will have recognised syndromes associated with impaired T cell development, such as complete DiGeorge syndrome or CHARGE syndrome. However a proportion of patients will have T cell lymphopaenia with no identifiable underlying aetiology10. Following identification of low or absent TREC by NBS, testing will be repeated and second-tier laboratory testing will be required.
Any child with a positive NBS for SCID should be discussed urgently with a paediatric immunologist at a tertiary centre.
Recommended initial investigations for a confirmed positive NBS are:
- Full blood count + differential
- Immunoglobulins
- Lymphocyte subsets – CD3/CD4/CD8/CD19/CD16/56
- Naïve CD4 and CD8 T cells – CD3/CD4/CD8/CD45RA/CD45RO
Additional second line investigations may be considered, including assessment for transplacental maternal engraftment (TME) and lymphocyte proliferation in response to phytohemagglutinin (PHA). Depending on the immunophenotype, other investigations may be useful such as assessment of the V-beta repertoire, fibroblast culture for radiosensitivity testing and consideration of measurement of adenosine deaminase (ADA) and purine nucleoside phosphorylase (PNP) enzyme levels and/or
metabolites. A microarray should also be considered to exclude 22q11 deletion/DiGeorge syndrome.
Figure 1: Diagnostic algorithm following positive NBS test10
Diagnosis in the absence of NBS
Any child suspected of having SCID should be immediately discussed with a paediatric immunologist at the closest tertiary paediatric institution. Further investigation to establish the diagnosis involves similar workup to that used following identification through NBS.
Rapid genetic testing is required to identify conditions that are not amenable to HSCT (e.g. thymic disorders), but also to identify those with radiosensitive conditions who will require a different approach to HSCT (e.g. DNA ligase IV). This may be best achieved through a commercially available panel or whole exome sequencing and should be performed in discussion with the specialist tertiary immunology centre.
Definitions of SCID
In the era of NBS, the definitions of typical SCID vs leaky SCID versus idiopathic T cell lymphopaenia are evolving. The definitions contained within this document are considered current best practice definitions of SCID from recent publications, following the introduction of NBS for SCID in the United States.
Typical SCID is characterised by absent or very low numbers of T cells (CD3+ T cells <300/uL) and no or very low T cell function (<10% lower range of normal), as measured by proliferation to PHA, or detectable TME 1, 5, 10-12. Mutations in recognised SCID genes are typically present. Atypical or leaky SCID is characterised by a reduced number of CD3+ T cells for age, less than 30% of lower limit of normal T cell function as measured by proliferation to PHA, reduced or absent naïve T cells and absence of TME 1, 5, 10-12. A subset of patients with leaky SCID and Omenn syndrome may have oligoclonal T cell expansion (limited T cell receptor [TCR] repertoire), generalised skin rash, elevated total Immunoglobulin E (IgE) and lymphadenopathy.
Patients with non-SCID T cell lymphopaenia may be identified by NBS, and typically have low T cells but naïve T cells are present (however may be low/absent), and ³30% the lower limit of normal lymphocyte proliferation in response to PHA (note this will be method dependent eg. whole blood versus separated PHA)10. They may also have other clinical features to suggest the diagnosis of a characterised syndrome associated with T cell lymphopaenia (e.g. DiGeorge, CHARGE).
Radiosensitive SCID (DNA repair defects) incorporates genetic deficiencies in multiple genes required for DNA repair. These patients typically present with a T-B-NK+ immunophenotype due to the dependence on these genes for the generation of a functional T and B cell antigen receptor during lymphocyte development. In addition, other clinical features may be present, including typical facies, microcephaly and developmental delay. These conditions are curable by HSCT, however early identification of radiosensitivity is essential to minimize radiation exposure prior to HSCT, to enable appropriate donor selection and choice of conditioning regimen10. Rapid genetic testing (e.g. Next Generation Sequencing [NGS] panel or targeted Sanger sequencing) may facilitate a diagnosis, however radiation sensitivity testing of patient fibroblasts may also be considered. Given the complexities of this group of patients, it is recommended that these patients are discussed at TAPID meetings on a case-by-case basis prior to proceeding to HSCT to assist with choice of conditioning regimen.
Supportive care for SCID patients prior to HSCT
The main aim of pre-transplant management for a patient diagnosed with SCID is to prevent infections that can have a detrimental impact on their outcome. The following recommendations are based on expert opinion as published literature on this topic is limited.
- Environment
- If a child with SCID needs admission to hospital they must be nursed in a single room with the door closed and follow local protective isolation guidelines. Ideally children with SCID should be managed in HEPA filtered rooms where available.
- Staff members caring for the child must be well and not have active cold sores (herpes labialis).
- Investigations should be performed within the room where possible. If the child needs to leave the room there should be minimum time spent waiting in areas with other patients present.
- Children with SCID may be managed out of hospital if they do not need acute hospital care, have rapid access to appropriate medical assessment if they become unwell and the home environment is deemed appropriate by the treating medical team.
- Measures should be taken to limit contact with family members e.g. siblings with active infections such as upper respiratory tract infection or active cold sores where possible.
- Prior to HSCT multiple medical reviews and investigations will be required. Timing of admission or relocation of the family close to the transplant centre should be discussed and determined on an individual basis. If remaining at home or in a local hospital regular review with the tertiary immunologist must be available, for example using telehealth.
- If the closest tertiary centre does not offer HSCT for SCID, early referral should be initiated to plan for transfer.
- Infection prophylaxis
- Children with SCID should be commenced on cotrimoxazole prophylaxis for Pneumocystis jirovecii (PJP) according to local policy from 4 weeks of age. If no local guideline exists 2.5mg/kg trimethoprim component twice daily three days per week is a suitable regime.
- If the patient is unable to tolerate co-trimoxazole, consider change to intravenous pentamidine 4mg/kg every 4 weeks or oral atovaquone as per AMH dosing (age dependent).
- Immunoglobulin replacement therapy (IRT) should be commenced with a starting dose 0.4-0.5g/kg/month. This can be given by either the intravenous (IVIg) or subcutaneous (SCIg) route. IRT should be commenced even when Immunoglobulin G (IgG) level is normal, as this is due to maternal transference and will not persist.
- Fluconazole should be commenced at prophylactic dosing, according to local protocol orAustralian Medicines Handbook (AMH) guidelines, suggested range 3 - 6mg/kg
- Every 72 hours if neonate <2 weeks of age
- Every 48 Hours if neonate 2-4 weeks of age
- Daily if >4 weeks of age
- Initiation of antifungal prophylaxis should commence at the time of diagnosis
- Consider itraconazole or an alternative mould active antifungal agent in those patients who are neutropenic
- Aciclovir prophylaxis should be considered if any carer with direct patient contact has a history of frequent cold sores.
- Recommended prophylactic aciclovir dose:
- Patients <6 months; 300mg/m2 three times a day orally
- Patients 6-24 months old; 100mg three times a day orally
- Consider administration of Palivizumab through winter months for respiratory syncytial virus (RSV) prophylaxis.
- Recommended prophylactic aciclovir dose:
- Management of infections
- Patients should undergo regular screening (every one to two weeks) for the following viruses by nucleic acid testing (PCR):
- Cytomegalovirus (CMV)
- Epstein Barr Virus(EBV)
- Adenovirus
- HHV6
- Note: Serological testing is unsuitable for patients with SCID
- Active infections (bacterial, fungal or viral) should be treated as per standard therapy, but SCID patients will not clear most infections without definitive (curative) therapy. The goal should be to stabilise and move to HSCT within an urgent timeframe.
- Infections may be prolonged or caused by atypical organisms. Consult local infectious diseases team for ongoing concerns.
- Patients may need to stay on anti-infective treatment (e.g. ganciclovir for CMV) until undergoing HSCT.
- Vaccination
- Children with SCID should not be given live vaccines, including rotavirus or BCG, due to the risk of active infection.
- When a diagnosis of SCID is suspected or confirmed, all vaccinations (live or inactive) should be deferred until after HSCT.
- Blood products
- Children with SCID should receive CMV negative, irradiated blood products.
- Breast feeding
- Mothers breast feeding or wishing to breast feed should have CMV serological status (IgG) checked. Antenatal CMV status, often taken in the first trimester is inadequate for decision making because of the risk of seroconversion in later pregnancy.
- All newly presenting infants should have CMV plasma polymerase chain reaction (PCR) tested urgently.
- If a mother is breast feeding at time of diagnosis, this should be stopped until mother and infant results available. The mother may continue to express milk.
- Regardless of infant CMV PCR result, if the mother is seronegative, breast feeding can be restarted. Advice should be provided to the mother regarding measures to reduce her risk of acquiring CMV such as that provided to pregnant women. https://beta.health.gov.au/resources/pregnancy-care-guidelines/part-g-targeted-maternal-health-tests/cytomegalovirus
- If the mother is seropositive and the infant is CMV PCR negative breast feeding should not be recommenced. There is no data about the safety of pasteurisation of breast milk in the setting of profound immune deficiency. Advice from a local midwifery team may be beneficial to manage the mother’s milk production.
- Due to the risk of bacterial contamination of powdered infant milk formula, use of ready-to-feed infant formula should be considered.
- Families should be advised that CMV can also be transmitted by saliva and other secretions so care should be taken to avoid accidental transmission (e.g. if a pacifier is used the parents shouldn't put the pacifier in their own mouth).
- If the mother is seropositive and the infant is CMV PCR positive, breast feeding should be encouraged/restarted.
- Supportive care
- Monitor height, weight and head circumference on a regular basis.
- Infants with SCID may present with failure to thrive and diarrhoea. Good nutrition is vital moving into HSCT. Involve the nutrition team and consider parenteral nutrition early if there are any concerns.
- Attention to skin care (in particular the nappy area in babies with diarrhoea) is crucial.
- Central venous access will be required for HSCT. Discuss with local surgeons and the transplant centre for their preferred form of access and timing of insertion.
- Urgent Human Leucocyte Antigen (HLA) typing for patient, parents and siblings should be undertaken at the earliest possible opportunity once a diagnosis of SCID is made
- Limit venepuncture to prevent iatrogenic anaemia.
- Haematology and biochemistry investigations are required only as clinically indicated.
- After initial diagnosis, immunological testing does not need to be monitored regularly unless there is a change in clinical picture. Consider repeating lymphocyte subsets if there are concerns about evolving maternal T cell engraftment or Omenn syndrome.
- Consider early referral to social work.
- Consider audiology and ophthalmology review as clinically indicated.
- Special considerations
- PEG-ADA; Enzyme replacement therapy (ERT) is available for adenosine deaminase deficiency (ADA SCID). This can be commenced whilst planning for curative therapy. Long term immune reconstitution is suboptimal with ERT and therefore, continuing treatment without definitive management is not recommended13,14.
Curative treatment for SCID
Haematopoietic Stem Cell Transplantation (HSCT)
Current standard of care for definitive correction of SCID is HSCT15, which must be performed urgently as outcomes are best when performed at an early age with no active infection15,16.
HSCT should only be undertaken in a specialist centre with suitable expertise and facilities to isolate and manage an infant with SCID.
Children with Omenn syndrome may require pre-transplant immunosuppression with agents such as corticosteroid and/or cyclosporine.
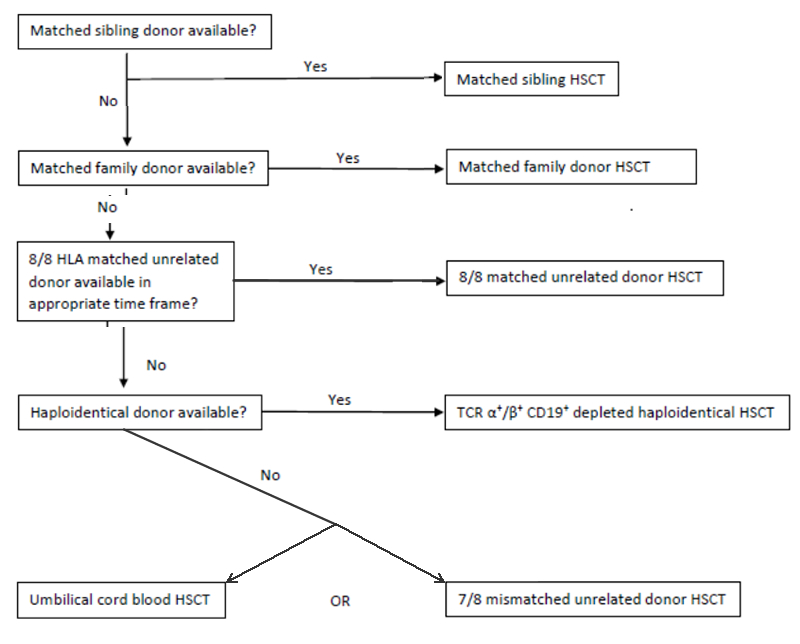
Donor selection
Choice of appropriate donor should be dependent on HLA match as well as donor availability, due to the urgent nature of HSCT. Donors should be high resolution typed at a minimum 6 loci (preferably 8-10), with the following selection algorithm observed.
Figure 2: Donor selection algorithm for SCID HSCT
Use of conditioning
Due to an absence of T cells, children with SCID can receive an unconditioned stem cell infusion from a genoidentical or haploidentical donor without risk of rejection. The resultant T cell function can be adequate to clear infection and allow survival. However, long term immune reconstitution is variable and B cell function will generally not recover17. Patients who receive conditioning prior to stem cell infusion have better long term immune reconstitution, particularly in regards to B cell engraftment and function. Use of conditioning also improves graft function and survival when using alternative donor sources18.
Alkylating agents form the backbone of conditioning for SCID patients, although they carry the risks of short and long term toxicity19-21. In the modern era, a treosulfan based regimen would be considered standard of care in centres where the drug was available. Treosulfan demonstrates good efficacy with less reported toxicity than busulfan22. Similarly, fludarabine has replaced cyclophosphamide as an immunosuppressive agent due to less toxicity in these patients with better long term T cell chimerism23. The combination of treosulfan and fludarabine has been successfully used in infants as young as two months of age22. It should be noted that the long term effects of treosulfan and fludarabine exposure in infants is unknown, however the TAPID group is of the considered opinion that poor graft function carries significant risks of long term morbidity and mortality, and hence warrants the use of conditioning in this age group.
Unconditioned infusion may be considered in patients who are unsuitable to receiving conditioning therapy, eg those with severe infections. Unconditioned infusion is not recommended in patients with Omenn syndrome or those with evidence of maternal engraftment.
Certain SCID subtypes present unique challenges in performing HSCT. Those with DNA repair defects (e.g. Artemis) are at increased risk of long term effects from alkylating agents, but these same patients are the most difficult to ensure sustained engraftment. Recent literature has demonstrated alkylator based conditioning therapy can result in good long term engraftment for these patients, but long term impacts need to be considered so that parents can make an informed decision19,24.
DNA ligase IV, Cernunnos-XLF and Nijmegen Breakage Syndrome require additional consideration due to their poor tolerance of alkylating agents. A suggested Fanconi style conditioning regime is available in the ESID-EBMT HSCT guidelines. It is recommended that when considering HSCT for these disorders a discussion regarding conditioning occurs within the TAPID group on a case-by-case basis.
The recommendations below are based on current published literature as well as consensus expert opinion.
Conditioning regimens for SCID^
|
Day |
Matched sibling donor* |
Matched unrelated donor/ Cord blood |
TCR αβ/CD19 depleted haplo* |
|
-10 |
ATG |
||
|
-9 |
ATG |
||
|
-8 |
ATG |
||
|
-7 |
FLU |
FLU |
FLU |
|
-6 |
FLU |
FLU |
FLU |
|
-5 |
TREO FLU |
TREO FLU |
TREO FLU |
|
-4 |
TREO FLU |
TREO FLU |
TREO FLU |
|
-3 |
TREO FLU Start CSA and MMF |
TREO FLU Start CSA and MMF |
TREO FLU |
|
-2 |
Rest day |
Rest day |
Start MMF |
|
-1 |
Rest day |
Rest day |
Rest day |
|
0 |
Donor cell infusion |
Donor cell infusion |
Donor cell infusion |
^ Not recommended for use in patients with DNA Ligase IV, Cernunnos-XLF, Nijmegen Breakage Syndrome.
*Consider addition of serotherapy for any patient with Omenn syndrome or evidence of maternal engraftment.
Alkylating agents used in conditioning
ATG - Anti-thymocyte globulin (Thymoglobulin) 2.5mg/kg/dose
CSA - Cyclosporin 2.5mg/kg bd
FLU - Fludarabine 30mg/m2/dose
MMF - Mycophenolate Mofetil 15mg/kg tds
TREO - Treosulfan 14g/m2 /dose (12g/m2 /dose if age<12 months, 10g/m2/dose if <4 months)
Gene therapy
Gene therapy is not currently available for patients in Australia or New Zealand. It is commercially available in Europe for ADA SCID. However, international trials are potentially available25 (Pai personal communication) to patients with other forms of SCID on a case by case basis. It is recommended clinicians seek input from the TAPID group before referring patients to any centre for gene therapy.
Thymic transplant
For thymic defects such as complete Di George syndrome or FOXN1 deficiency, thymic transplant should be considered as an alternative to HSCT. It is not currently available in Australia or New Zealand but is available in international trials. It is recommended clinicians seek input from the TAPID group on a case by case basis.
Appendices
Classification of SCID based on molecular defect2
|
Type of defect |
Specific molecular abnormality |
|
Cytokine signalling defects |
Common γ chain Janus Kinase 3 (JAK3) Interleukin 7 receptor α (IL-7α) |
|
T cell receptor (TCR) defects |
CD3δ/ε/ζ ζ associated protein-70 (ZAP-70) Lymphocyte specific protein tyrosine kinase (lck) ORAI calcium release-activated calcium modulator (Orai1) |
|
VDJ recombination defects |
Recombinase activating gene 1 and 2 (RAG1/2) Artemis DNA ligase IV Cernunnos-X-ray cross complementing gene 4-like factor (XLF) DNA protein kinase catalytic subunit (DNA PKcs) |
|
Defects of metabolism |
Adenosine deaminase deficiency (ADA) Adenylate kinase 2 (AK2, reticular dysgenesis) |
|
Other |
CD45 Coronin 1A RNA component of mitochondrial RNA processing endoribonuclease (RMRP), Nude SCID (FOXN1) |
SCID Immunophenotypes2
|
T-B+NK- |
Common γ chain JAK3 |
|
T-B+NK+ |
IL-7α CD3δ/ε/ζ Coronin 1A ZAP-70 CD45 |
|
T-B-NK- |
ADA deficiency Reticular dysgenesis |
|
T-B-NK+ |
RAG1/2 Artemis DNA ligase IV Cernunnos-XLF DNA PKcs |
Omenn’s syndrome
Omenn’s syndrome is typically caused by RAG1/2, but can be a result of many different gene mutations26,27. It is clinically characterised by failure to thrive, opportunistic infection, severe infiltrative erythrodermatous rash, lymphadenopathy and hepatosplenomegaly. Immunologically these patients have an expansion of autologous dysregulated T cells and poor humoral immunity.
References
- Shearer WT, Dunn E, Notarangelo LD, et al. Establishing diagnostic criteria for severe combined immunodeficiency disease (SCID), leaky SCID, and Omenn syndrome: the Primary Immune Deficiency Treatment Consortium experience. J Allergy Clin Immunol. 2014;133(4):1092-1098.
- Gaspar HB, Qasim W, Davies EG, Rao K, Amrolia PJ, Veys P. How I treat severe combined immunodeficiency. Blood. 2013;122(23):3749-3758.
- Bonilla FA, Khan DA, Ballas ZK, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol. 2015;136(5):1186-1205 e1181-1178.
- van der Burg M, Gennery AR. Educational paper. The expanding clinical and immunological spectrum of severe combined immunodeficiency. Eur J Pediatr. 2011;170(5):561-571.
- Kwan A, Abraham RS, Currier R, et al. Newborn screening for severe combined immunodeficiency in 11 screening programs in the United States. JAMA. 2014;312(7):729-738.
- Peckham CS, Johnson C, Ades A, Pearl K, Chin KS. Early acquisition of cytomegalovirus infection. Arch Dis Child. 1987;62(8):780-785.
- Australian Bureau of Statistics.
- Puck JM. Laboratory technology for population-based screening for severe combined immunodeficiency in neonates: the winner is T-cell receptor excision circles. J Allergy Clin Immunol. 2012;129(3):607-616.
- Puck JM. Neonatal screening for severe combined immunodeficiency. Curr Opin Pediatr. 2011;23(6):667-673.
- Dorsey MJ, Dvorak CC, Cowan MJ, Puck JM. Treatment of infants identified as having severe combined immunodeficiency by means of newborn screening. J Allergy Clin Immunol. 2017;139(3):733-742.
- Kwan A, Puck JM. History and current status of newborn screening for severe combined immunodeficiency. Semin Perinatol. 2015;39(3):194-205.
- Kwan A, Church JA, Cowan MJ, et al. Newborn screening for severe combined immunodeficiency and T-cell lymphopenia in California: results of the first 2 years. J Allergy Clin Immunol. 2013;132(1):140-150.
- Chan B, Wara D, Bastian J, et al. Long-term efficacy of enzyme replacement therapy for adenosine deaminase (ADA)-deficient severe combined immunodeficiency (SCID). Clin Immunol. 2005;117(2):133-143.
- Hassan A, Booth C, Brightwell A, et al. Outcome of hematopoietic stem cell transplantation for adenosine deaminase-deficient severe combined immunodeficiency. Blood. 2012;120(17):3615-3624; quiz 3626.
- Pai SY, Logan BR, Griffith LM, et al. Transplantation outcomes for severe combined immunodeficiency, 2000-2009. N Engl J Med. 2014;371(5):434-446.
- Dvorak CC, Puck JM, Wahlstrom JT, Dorsey M, Melton A, Cowan MJ. Neurologic event-free survival demonstrates a benefit for SCID patients diagnosed by newborn screening. Blood Adv. 2017;1(20):1694-1698.
- Dvorak CC, Hassan A, Slatter MA, et al. Comparison of outcomes of hematopoietic stem cell transplantation without chemotherapy conditioning by using matched sibling and unrelated donors for treatment of severe combined immunodeficiency. J Allergy Clin Immunol. 2014;134(4):935-943 e915.
- Fernandes JF, Rocha V, Labopin M, et al. Transplantation in patients with SCID: mismatched related stem cells or unrelated cord blood? Blood. 2012;119(12):2949-2955.
- Schuetz C, Neven B, Dvorak CC, et al. SCID patients with ARTEMIS vs RAG deficiencies following HCT: increased risk of late toxicity in ARTEMIS-deficient SCID. Blood. 2014;123(2):281-289.
- Cole BO, Welbury RR, Bond E, Abinun M. Dental manifestations in severe combined immunodeficiency following bone marrow transplantation. Bone Marrow Transplant. 2000;25(9):1007-1009.
- Slatter MA, Gennery AR, Cheetham TD, et al. Thyroid dysfunction after bone marrow transplantation for primary immunodeficiency without the use of total body irradiation in conditioning. Bone Marrow Transplant. 2004;33(9):949-953.
- Slatter MA, Rao K, Abd Hamid IJ, et al. Treosulfan and Fludarabine Conditioning for Hematopoietic Stem Cell Transplantation in Children with Primary Immunodeficiency: UK Experience. Biol Blood Marrow Transplant. 2018;24(3):529-536.
- Slatter MA, Rao K, Amrolia P, et al. Treosulfan-based conditioning regimens for hematopoietic stem cell transplantation in children with primary immunodeficiency: United Kingdom experience. Blood. 2011;117(16):4367-4375.
- Cowan MJ, Gennery AR. Radiation-sensitive severe combined immunodeficiency: The arguments for and against conditioning before hematopoietic cell transplantation--what to do? J Allergy Clin Immunol. 2015;136(5):1178-1185.
- Cicalese MP, Ferrua F, Castagnaro L, et al. Update on the safety and efficacy of retroviral gene therapy for immunodeficiency due to adenosine deaminase deficiency. Blood. 2016;128(1):45-54.
- Gennery AR, Slatter MA, Rice J, et al. Mutations in CHD7 in patients with CHARGE syndrome cause T-B + natural killer cell + severe combined immune deficiency and may cause Omenn-like syndrome. Clin Exp Immunol. 2008;153(1):75-80.
- Villa A, Santagata S, Bozzi F, et al. Partial V(D)J recombination activity leads to Omenn syndrome. Cell. 1998;93(5):885-896.
© ASCIA 2019
ASCIA is the peak professional body of clinical immunology/allergy specialists in Australia and New Zealand.
ASCIA resources are based on published literature and expert review, however, they are not intended to replace medical advice. The content of ASCIA resources is not influenced by any commercial organisations.
For more information go to www.allergy.org.au
To donate to immunology/allergy research go to www.allergyimmunology.org.au