ASCIA Guidelines - Adrenaline (Epinephrine) Injector Prescription
![]() ASCIA Guidelines for adrenaline injector prescription 2024127.88 KB
ASCIA Guidelines for adrenaline injector prescription 2024127.88 KB
This document has been developed by ASCIA, the peak professional body of clinical immunology/allergy specialists in Australia and New Zealand. ASCIA information is based on published literature and expert review, is not influenced by commercial organisations and is not intended to replace medical advice. For patient or carer support contact Allergy & Anaphylaxis Australia or Allergy New Zealand
The aim of these guidelines is to outline the appropriate prescription and dose of adrenaline (epinephrine) injectors for use in non-medical settings, for the emergency first aid treatment of potentially life-threatening severe allergic reactions (anaphylaxis).
These guidelines should be used in conjunction with clinical judgement, with consideration of issues that include training on a new device and preference of device from the patient or their carer.
These guidelines include adrenaline injector devices that are currently available in Australia and New Zealand, and are based on published evidence regarding:
- Recommended doses of adrenaline injectors to treat severe symptoms, reduce the risk of serious morbidity (e.g. cerebrovascular damage) and prevent fatalities due to anaphylaxis.
- Epidemiology of allergic reactions triggers that may be difficult to avoid.
- Risk factors that have been associated with fatal anaphylaxis.
ASCIA Adrenaline Injector Recommendations
Adrenaline rapidly reverses the effects of anaphylaxis and adrenaline injector devices are considered to be first line emergency first aid treatment for anaphylaxis.
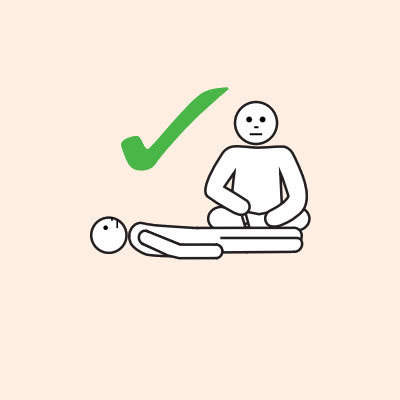
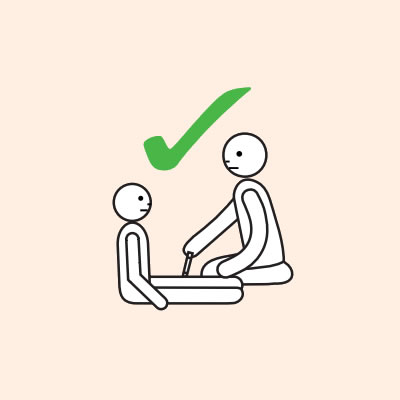
Each adrenaline injector device contains a single fixed dose of adrenaline that is administered intramuscularly into the outer mid-thigh, for safe and rapid absorption of adrenaline.
Adrenaline ampoules and syringes are not considered by ASCIA to be suitable for non-medical settings such as schools, children’s education/care centres and workplaces.
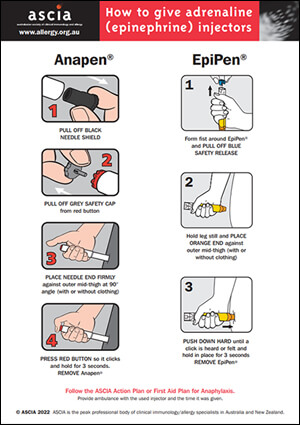
ASCIA recommends the use of adrenaline as the first line treatment for anaphylaxis using either of the following two brands of adrenaline injectors:
- EpiPen® Jr (150 microgram) and EpiPen® (300 microgram) - TGA approved, available in Australia on the PBS (up to two devices per prescription), and New Zealand (Pharmac listed 2023).
- Anapen® 500 (500 microgram) - TGA approved and available in Australia on the PBS (up to two devices per prescription).
It is important to specify brand and tick box on PBS prescription to ensure that brand is not substituted. EpiPen® and Anapen® are also available without prescription.
Both EpiPen® and Anapen® devices are widely used in other countries. Most countries have multiple brands of adrenaline injector devices available, and this is important for the following reasons:
- To ensure continued supply of life saving adrenaline, particularly if one brand has stock shortages.
- To provide doctors with a choice of dose, who may prefer to prescribe a higher dose (500 microgram device) for people over 50kg .
- A 500 microgram dose can potentially prevent the need for further doses of adrenaline (which is important due to increasing ambulance delays and many people only carrying one device).
- To encourage suppliers to provide devices with longer shelf life.
- To provide choice for consumers to access devices with points of difference to best suit their needs.
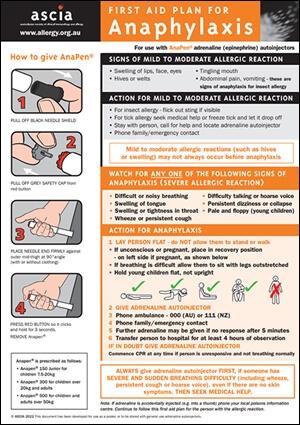
It is essential that patients and carers are trained to use the prescribed device (EpiPen® or Anapen®) and provided with an ASCIA Action Plan for Anaphylaxis, including the prescribed device instructions.
ASCIA Adrenaline Injector Dose Recommendations
ASCIA recommends the adrenaline injector doses listed below, based on expert consensus and standard practice by ASCIA members, which vary to the product information. These doses are consistent with the Acute Anaphylaxis Clinical Care Standard for Australia, Australian Prescriber Anaphylaxis Wallchart, Australian Immunisation Handbook and international recommendations from the World Allergy Organisation (WAO), Canada and the UK (refer to weblinks below).
Children 7.5-20kg (aged around one to five years): EpiPen® Jr (150 microgram)
Children over 20kg and adults: EpiPen® (300 microgram)
Children and adults over 50kg: Anapen® 500 (500 microgram)*
*The dose of adrenaline in Anapen 500 is consistent with the intramuscular injection (IMI) dose recommendations for people who weigh more than 50kg in the publications listed below:
https://www.worldallergyorganizationjournal.org/article/S1939-4551(20)30375-6/fulltext
https://www.resuscitationjournal.com/article/S0300-9572(21)00150-7/fulltext
https://onlinelibrary.wiley.com/doi/full/10.1111/cea.14055
Children less than 7.5kgAdrenaline injectors are not usually recommended for children less than 7.5kg as the risk of fatal anaphylaxis in children this age is very low. While the ‘optimal’ dose of adrenaline is unknown there is a risk that the lower dose adrenaline injector could provide a significant overdose. ASCIA does not recommend the use of adrenaline ampoules and syringes for children less than 7.5kg as they are inappropriate for non-medical settings, such as childcare centres. Even if ampoules are administered by ‘trained’ non-medical personnel, such as parents, there may be a risk of a serious dosing error. If there is a concern regarding an infant under 7.5kg requiring adrenaline, they should be referred to a clinical immunology/allergy specialist for assessment. The issues should be discussed with the parents based on a risk assessment. Where it is felt that it is essential for emergency adrenaline to be prescribed for a child less than 7.5kg, the risk of administering a ‘fixed’ overdose via a 150 microgram device is considered to be lower than the risk of a dose error with an adrenaline ampoule and syringe. |
ASCIA Adrenaline Injector Prescription Guide
Adrenaline injector prescription is recommended for patients with:
|
Adrenaline injector prescription is sometimes recommended for patients with a history of a generalised allergic reaction, with one or more of the following additional risk factors:
This list is not comprehensive and if there is a concern, patients should be referred to a clinical immunology/allergy specialist for assessment.
These factors should be considered when deciding whether an adrenaline autoinjector is prescribed, as they are known risk factors for more severe or fatal allergic reactions. |
Adrenaline injector prescription is not normally recommended as follows:
|
To access clinical definitions refer to the ASCIA Guidelines for Acute Management of Anaphylaxis on the ASCIA website www.allergy.org.au/hp/papers/acute-management-of-anaphylaxis-guidelines
Updated ASCIA Action Plans for Anaphylaxis, e-training courses and other resources which include EpiPen® and Anapen® instructions are available on the ASCIA website www.allergy.org.au/hp/anaphylaxis
Anaphylaxis Management Plan
An adrenaline injector should only be prescribed within the context of a comprehensive Anaphylaxis Management Plan that includes the following:
1. Referral to a clinical immunology/allergy specialist
Review by a clinical immunology/allergy specialist should occur to:
- Ascertain if the correct trigger(s) have been identified.
- Determine whether the allergy persists.
- Advise on specific management, including suitability for allergen immunotherapy (if available).
- Advise on co-factors that may increase the risk of more serious reactions (e.g. use of beta blockers, NSAID, exercise, asthma control).
2. Identification of anaphylaxis trigger(s)
This should include a comprehensive history, clinical examination, appropriate use and interpretation of allergy testing and under some circumstances, deliberate challenge to prove or disprove allergy.
3. Education on the avoidance of trigger(s)
This is particularly important with food allergy induced anaphylaxis.
4. Provision of an ASCIA Action Plan for Anaphylaxis (emergency response plan)
This plan includes:
- Personal details - patient’s photo, name, confirmed allergens and family/emergency contact.
- Symptoms and signs indicating when to use the adrenaline injector.
- Instructions on how to use the adrenaline injector.
5. Appropriate follow-up
Review by a patient’s medical practitioner (normally their GP) approximately 12-18 monthly should occur to:
- Review any allergic reactions that have occurred since their last review.
- Examine co-factors (such as poorly controlled or persistent asthma) that may increase the risk of more serious reactions.
- Prescribe adrenaline injectors. If you do not want brand substitution, it is important to specify brand and tick box on PBS prescription.
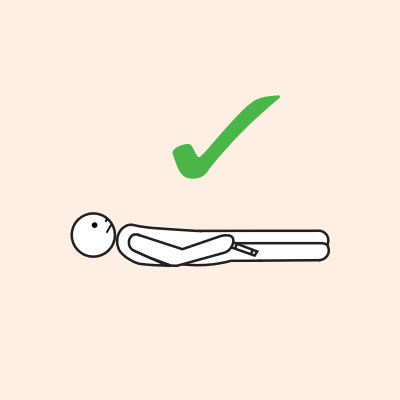
- Provide re-education on adrenaline injector use (using a trainer device of the prescribed brand) and positioning (e.g. not standing or walking when experiencing anaphylaxis).
- Renew ASCIA Action Plan for Anaphylaxis - the general version includes instructions for EpiPen® and Anapen®..
- Provide ASCIA Travel Plan if required.
- Determine if specialist review is required to ascertain if the allergy persists, new allergies have developed or if more detailed revi4ew is required.
© ASCIA 2024
Content and weblinks updated July 2024
For more information go to www.allergy.org.au/hp/anaphylaxis
To support allergy and immunology research go to www.allergyimmunology.org.au/donate


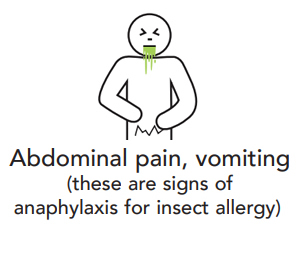
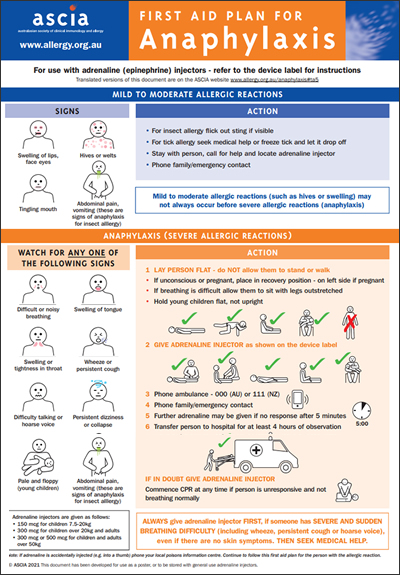
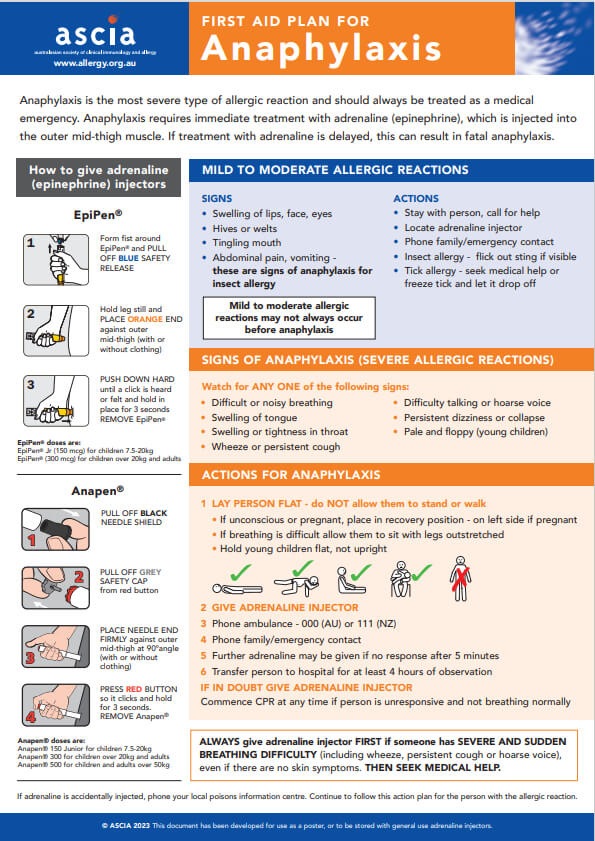 Anaphylaxis is the most severe type of allergic reaction and should always be treated as a medical emergency. Anaphylaxis requires immediate treatment with adrenaline (epinephrine), which is injected into the outer mid-thigh muscle. If treatment with adrenaline is delayed, this can result in fatal anaphylaxis.
Anaphylaxis is the most severe type of allergic reaction and should always be treated as a medical emergency. Anaphylaxis requires immediate treatment with adrenaline (epinephrine), which is injected into the outer mid-thigh muscle. If treatment with adrenaline is delayed, this can result in fatal anaphylaxis.